FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Cayston Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
CAYSTON® is indicated to improve respiratory symptoms in cystic fibrosis (CF) patients with Pseudomonas aeruginosa. Safety and effectiveness have not been established in pediatric patients below the age of 7 years, patients with FEV1 <25% or >75% predicted, or patients colonized with Burkholderia cepacia [see Clinical Studies (14)].
To reduce the development of drug-resistant bacteria and maintain the effectiveness of CAYSTON and other antibacterial drugs, CAYSTON should be used only to treat patients with CF known to have Pseudomonas aeruginosa in the lungs.
History
There is currently no drug history available for this drug.
Other Information
A dose of CAYSTON consists of a 2 mL amber glass vial containing lyophilized aztreonam (75 mg) and lysine (46.7 mg), and a low-density polyethylene ampule containing 1 mL sterile diluent (0.17% sodium chloride). The reconstituted solution is for inhalation. The formulation contains no preservatives or arginine.
The active ingredient in CAYSTON is aztreonam, a monobactam antibacterial. The monobactams are structurally different from beta-lactam antibiotics (e.g., penicillins, cephalosporins, carbapenems) due to a monocyclic nucleus. This nucleus contains several side chains; sulfonic acid in the 1-position activates the nucleus, an aminothiazolyl oxime side chain in the 3-position confers specificity for aerobic Gram-negative bacteria including Pseudomonas spp., and a methyl group in the 4-position enhances beta-lactamase stability.
Aztreonam is designated chemically as (Z)-2-[[[(2-amino-4-thiazolyl)[[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]carbamoyl]methylene]amino]oxy]-2-methylpropionic acid. The structural formula is presented below:

CAYSTON is a white to off-white powder. CAYSTON is sterile, hygroscopic, and light sensitive. Once reconstituted with the supplied diluent, the pH range is 4.5 to 6.0.
Sources
Cayston Manufacturers
-
Gilead Sciences, Inc.
![Cayston (Aztreonam) Kit [Gilead Sciences, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Cayston | Gilead Sciences, Inc.
![Cayston (Aztreonam) Kit [Gilead Sciences, Inc.] Cayston (Aztreonam) Kit [Gilead Sciences, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
2.1 Dosing InformationThe recommended dose of CAYSTON for both adults and pediatric patients 7 years of age and older is one single-use vial (75 mg of aztreonam) reconstituted with 1 mL of sterile diluent administered 3 times a day for a 28-day course (followed by 28 days off CAYSTON therapy). Dosage is not based on weight or adjusted for age. Doses should be taken at least 4 hours apart.
CAYSTON is administered by inhalation using an Altera® Nebulizer System. Patients should use a bronchodilator before administration of CAYSTON.
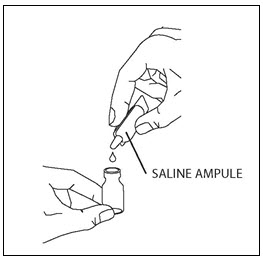
2.2 Instructions for CAYSTON ReconstitutionCAYSTON should be administered immediately after reconstitution. Do not reconstitute CAYSTON until ready to administer a dose.
Take one amber glass vial containing CAYSTON and one diluent ampule from the carton. To open the glass vial, carefully remove the metal ring by lifting or pulling the tab and remove the gray rubber stopper. Twist the tip off the diluent ampule and squeeze the liquid into the glass vial. Replace the rubber stopper, then gently swirl the vial until contents have completely dissolved.
The empty vial, stopper, and diluent ampule should be disposed of properly upon completion of dosing.
2.3 Instructions for CAYSTON AdministrationCAYSTON is administered by inhalation using an Altera Nebulizer System. CAYSTON should not be administered with any other nebulizer. CAYSTON should not be mixed with any other drugs in the Altera Nebulizer Handset.
CAYSTON is not for intravenous or intramuscular administration.
Patients should use a bronchodilator before administration of CAYSTON. Short-acting bronchodilators can be taken between 15 minutes and 4 hours prior to each dose of CAYSTON. Alternatively, long-acting bronchodilators can be taken between 30 minutes and 12 hours prior to administration of CAYSTON. For patients taking multiple inhaled therapies, the recommended order of administration is as follows: bronchodilator, mucolytics, and lastly, CAYSTON.
To administer CAYSTON, pour the reconstituted solution into the handset of the nebulizer system. Turn the unit on. Place the mouthpiece of the handset in your mouth and breathe normally only through your mouth. Administration typically takes between 2 and 3 minutes. Further patient instructions on how to administer CAYSTON are provided in the FDA-approved patient labeling. Instructions on testing nebulizer functionality and cleaning the handset are provided in the Instructions for Use included with the nebulizer system.
Login To Your Free Account