FDA records indicate that there are no current recalls for this drug.
Lingerie De Peau Bb Invisible Skin-fusion Multi-perfecting Makeup With Sunscreen Broad Spectrum Spf 30 Medium
Are you a medical professional?
Trending Topics
Lingerie De Peau Bb Invisible Skin-fusion Multi-perfecting Makeup With Sunscreen Broad Spectrum Spf 30 Medium Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Testosterone gel is indicated for replacement therapy in adult males for conditions associated with a deficiency or absence of endogenous testosterone:
- Primary hypogonadism (congenital or acquired): testicular failure due to cryptorchidism, bilateral torsion, orchitis, vanishing testis syndrome, orchiectomy, Klinefelter's syndrome, chemotherapy, or toxic damage from alcohol or heavy metals. These men usually have low serum testosterone levels and gonadotropins (follicle-stimulating hormone [FSH], luteinizing hormone [LH]) above the normal range.
- Hypogonadotropic hypogonadism (congenital or acquired): gonadotropin or luteinizing hormone-releasing hormone (LHRH) deficiency or pituitary-hypothalamic injury from tumors, trauma, or radiation. These men have low testosterone serum levels but have gonadotropins in the normal or low range.
Limitations of Use:
- Safety and efficacy of testosterone gel in men with "age-related hypogonadism" (also referred to as "late-onset hypogonadism") have not been established.
- Safety and efficacy of testosterone gel in males less than 18 years old have not been established [see Use in Specific Populations (8.4)].
- Topical testosterone products may have different doses, strengths, or application instructions that may result in different systemic exposure [see Dosage and Administration (2) and Clinical Pharmacology (12.3)].
History
There is currently no drug history available for this drug.
Other Information
Testosterone gel, for topical use is a clear to translucent hydroalcoholic topical gel containing testosterone, an androgen. Testosterone gel provides continuous transdermal delivery of testosterone for 24 hours, following a single application to intact, clean, dry skin of the shoulders and/or upper arms.
Testosterone gel is available in unit-dose tubes, unit-dose packets, and a metered-dose pump. One 5-g or two 5-g tubes/packets of testosterone gel contains 50 mg or 100 mg of testosterone, respectively. One pump actuation dispenses 1.25 g of gel, which contains 12.5 mg of testosterone. Four pump actuations or eight pump actuations contain 50 mg or 100 mg of testosterone, respectively. Each metered-dose pump container is capable of dispensing 60 pump actuations.
The active pharmacological ingredient in testosterone gel is testosterone. Testosterone USP is a white to practically white crystalline powder chemically described as 17-β hydroxyandrost-4-en-3-one. The structural formula is shown in the following figure:
Testosterone (C19H28O2) MW: 288.42
Inactive ingredients in testosterone gel are carbomer copolymer Type B, carbomer homopolymer Type C, diisopropyl adipate, ethyl alcohol, glycerin, methyl laurate, oleyl alcohol, polyethylene glycol, propylene glycol, purified water, and tromethamine.
Sources
Lingerie De Peau Bb Invisible Skin-fusion Multi-perfecting Makeup With Sunscreen Broad Spectrum Spf 30 Medium Manufacturers
-
Guerlain S.a.
![Lingerie De Peau Bb Invisible Skin-fusion Multi-perfecting Makeup With Sunscreen Broad Spectrum Spf 30 Medium (Octinoxate, Oxybenzone, Titanium Dioxide) Cream [Guerlain S.a.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Lingerie De Peau Bb Invisible Skin-fusion Multi-perfecting Makeup With Sunscreen Broad Spectrum Spf 30 Medium | Upsher-smith Laboratories, Inc.
![Lingerie De Peau Bb Invisible Skin-fusion Multi-perfecting Makeup With Sunscreen Broad Spectrum Spf 30 Medium (Octinoxate, Oxybenzone, Titanium Dioxide) Cream [Guerlain S.a.] Lingerie De Peau Bb Invisible Skin-fusion Multi-perfecting Makeup With Sunscreen Broad Spectrum Spf 30 Medium (Octinoxate, Oxybenzone, Titanium Dioxide) Cream [Guerlain S.a.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Prior to initiating testosterone gel, confirm the diagnosis of hypogonadism by ensuring that serum testosterone concentrations have been measured in the morning on at least two separate days and that these serum testosterone concentrations are below the normal range.
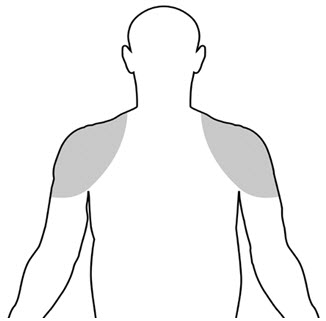
2.1 Dosing and Dose AdjustmentThe recommended starting dose of testosterone gel is 50 mg of testosterone (one tube, one packet, or 4 pump actuations) applied topically once daily at approximately the same time each day to clean, dry intact skin of the shoulders and/or upper arms.
Dose Adjustment
To ensure proper dosing, serum testosterone concentrations should be measured. Morning, pre-dose serum testosterone concentrations should be measured approximately 14 days after initiation of therapy to ensure proper serum testosterone concentrations are achieved. If the serum testosterone concentration is below the normal range (300 ng/dL to 1,000 ng/dL), the daily testosterone gel dose may be increased from 50 mg testosterone (one tube, one packet, or 4 pump actuations) to 100 mg of testosterone (two tubes, two packets, or 8 pump actuations) once daily.
The maximum recommended dose of testosterone gel is 100 mg once daily.
2.2 Administration InstructionsUnit-Dose Tube or Packet
Upon opening the tube or packet the entire contents should be squeezed into the palm of the hand and immediately applied to the shoulders and/or upper arms (area of application should be limited to the area that will be covered by the patient's short sleeve t-shirt [see figure below]).
Table 1 has specific dosing guidelines for when the unit-dose tubes or packets are used.
Table 1: Specific Dosing Guideline for Using the Unit-Dose Tubes or Packets Prescribed Daily Dose Number of Unit-Dose Tubes or Packets Application Method 50 mg testosterone One unit-dose tube or packet (once daily) Apply one unit-dose tube or packet to one upper arm and shoulder. 100 mg testosterone Two unit-dose tubes or packets (once daily) Apply one unit-dose tube or packet to one upper arm and shoulder and then apply one unit-dose tube or packet to the opposite upper arm and shoulder.Multi-Dose Metered Pump
Patients should be instructed to prime the pump before using it for the first time by fully depressing the pump mechanism (actuation) 3 times and discard this portion of the product to assure precise dose delivery. After the priming procedure, patients should completely depress the pump one time (actuation) for every 12.5 mg of testosterone required to achieve the daily prescribed dosage. Table 2 has specific dosing guidelines for when the metered pump is used.
Table 2: Specific Dosing Guidelines for Using the Multi-Dose Pump Prescribed Daily Dose Number of Pump Actuations Application Method 50 mg testosterone 4 (once daily) Apply 4 pump actuations to one upper arm and shoulder 100 mg testosterone 8 (once daily) Apply 4 pump actuations to one upper arm and shoulder and then apply 4 pump actuations to the opposite upper arm and shoulderThe prescribed amount of product should be delivered directly into the palm of the hand and immediately applied to the shoulders and/or upper arms (area of application should be limited to the area that will be covered by the patient's short sleeve t-shirt [see figure below]).
Do not apply testosterone gel to the genitals or to the abdomen.
Application sites should be allowed to dry completely prior to dressing.
Hands should be washed thoroughly with soap and water after testosterone gel has been applied.
Avoid fire, flame or smoking during the application of testosterone gel until the gel has dried [see Warnings and Precautions (5.2, 5.15)].
In order to prevent transfer to another person, clothing should be worn to cover the application sites. If direct skin-to-skin contact of the application site(s) with another person is anticipated, the application sites must be washed thoroughly with soap and water [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)].
The patient should avoid swimming or showering or washing the administration site for a minimum of 2 hours after application [see Clinical Pharmacology (12.3)].
Strict adherence to the following precautions is advised in order to minimize the potential for secondary exposure to testosterone from testosterone gel treated skin:
Children and women should avoid contact with unwashed or unclothed application site(s) of men using testosterone gel. Testosterone gel should only be applied to the upper arms and shoulders. The area of application should be limited to the area that will be covered by a short sleeve t-shirt. Patients should wash their hands with soap and water immediately after applying testosterone gel. Patients should cover the application site(s) with clothing (e.g., a t-shirt) after the gel has dried. Prior to any situation in which direct skin-to-skin contact of the application site(s) with another person is anticipated, patients should wash the application site(s) thoroughly with soap and water to remove any testosterone residue. In the event that unwashed or unclothed skin to which testosterone gel has been applied comes in direct contact with the skin of another person, the general area of contact on the other person should be washed with soap and water as soon as possible.
Login To Your Free Account