FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Stelara Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
STELARA® is indicated for the treatment of adult patients (18 years or older) with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy.
STELARA® is indicated for the treatment of adult patients (18 years or older) with active psoriatic arthritis. STELARA® can be used alone or in combination with methotrexate (MTX).
History
There is currently no drug history available for this drug.
Other Information
STELARA® is a human IgG1κ monoclonal antibody against the p40 subunit of the IL-12 and IL-23 cytokines. Using DNA recombinant technology, STELARA® is produced in a well characterized recombinant cell line and is purified using standard bio-processing technology. The manufacturing process contains steps for the clearance of viruses. STELARA® is comprised of 1326 amino acids and has an estimated molecular mass that ranges from 148,079 to 149,690 Daltons.
STELARA®, for subcutaneous use, is available as: 45 mg of ustekinumab in 0.5 mL and 90 mg of ustekinumab in 1 mL. STELARA® is supplied as a sterile solution in a single-use prefilled syringe with a 27 gauge fixed ½ inch needle, or a single-use 2 mL Type I glass vial with a coated stopper. The syringe is fitted with a passive needle guard and a needle cover that is manufactured using a dry natural rubber (a derivative of latex).
Each 45 mg ustekinumab prefilled syringe also contains: L-histidine and L-histidine monohydrochloride monohydrate (0.5 mg), Polysorbate 80 (0.02 mg), and sucrose (38 mg) to fill to a final volume of 0.5 mL.
Each 90 mg ustekinumab prefilled syringe also contains: L-histidine and L-histidine monohydrochloride monohydrate (1 mg), Polysorbate 80 (0.04 mg), and sucrose (76 mg) to fill to a final volume of 1 mL.
Each 45 mg ustekinumab vial also contains: L-histidine and L-histidine monohydrochloride monohydrate (0.5 mg), Polysorbate 80 (0.02 mg), and sucrose (38 mg) to fill to a final volume of 0.5 mL.
Each 90 mg ustekinumab vial also contains: L-histidine and L-histidine monohydrochloride monohydrate (1 mg), Polysorbate 80 (0.04 mg), and sucrose (76 mg) to fill to a final volume of 1 mL.
The STELARA® solution is colorless to slightly yellow in appearance and has a pH of 5.7–6.3. STELARA® does not contain preservatives.
Sources
Stelara Manufacturers
-
Janssen Biotech, Inc.
![Stelara (Ustekinumab) Injection, Solution [Janssen Biotech, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Stelara | Janssen Biotech, Inc.
![Stelara (Ustekinumab) Injection, Solution [Janssen Biotech, Inc.] Stelara (Ustekinumab) Injection, Solution [Janssen Biotech, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
2.1 DosingSTELARA® is administered by subcutaneous injection.
Psoriasis
For patients weighing ≤100 kg (220 lbs), the recommended dose is 45 mg initially and 4 weeks later, followed by 45 mg every 12 weeks. For patients weighing >100 kg (220 lbs), the recommended dose is 90 mg initially and 4 weeks later, followed by 90 mg every 12 weeks.In subjects weighing >100 kg, 45 mg was also shown to be efficacious. However, 90 mg resulted in greater efficacy in these subjects [see Clinical Studies (14)].
Psoriatic Arthritis
The recommended dose is 45 mg initially and 4 weeks later, followed by 45 mg every 12 weeks. For patients with co-existent moderate-to-severe plaque psoriasis weighing >100 kg (220 lbs), the recommended dose is 90 mg initially and 4 weeks later, followed by 90 mg every 12 weeks. 2.2 General Considerations for AdministrationSTELARA® is for subcutaneous administration. STELARA® is intended for use under the guidance and supervision of a physician. STELARA® should only be administered to patients who will be closely monitored and have regular follow-up visits with a physician.
After proper training in subcutaneous injection technique, a patient may self inject with STELARA® if a physician determines that it is appropriate. Patients should be instructed to follow the directions provided in the Medication Guide (see Medication Guide).
Prior to administration, STELARA® should be visually inspected for particulate matter and discoloration. STELARA® is colorless to light yellow and may contain a few small translucent or white particles. STELARA® should not be used if it is discolored or cloudy, or if other particulate matter is present. STELARA® does not contain preservatives; therefore, any unused product remaining in the vial and/or syringe should be discarded.
The needle cover on the prefilled syringe contains dry natural rubber (a derivative of latex). The needle cover should not be handled by persons sensitive to latex.
It is recommended that each injection be administered at a different anatomic location (such as upper arms, gluteal regions, thighs, or any quadrant of abdomen) than the previous injection, and not into areas where the skin is tender, bruised, erythematous, or indurated. When using the single-use vial, a 27 gauge, ½ inch needle is recommended.
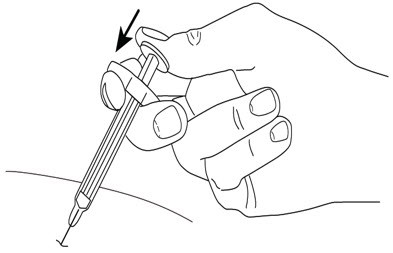
2.3 Instructions for Administration of STELARA® Prefilled Syringes Equipped with Needle Safety GuardRefer to the diagram below for the provided instructions.
To prevent premature activation of the needle safety guard, do not touch the NEEDLE GUARD ACTIVATION CLIPS at any time during use.
Hold the BODY and remove the NEEDLE COVER. Do not hold the PLUNGER or PLUNGER HEAD while removing the NEEDLE COVER or the PLUNGER may move. Do not use the prefilled syringe if it is dropped without the NEEDLE COVER in place. Inject STELARA® subcutaneously as recommended [see Dosage and Administration (2.2)]. Inject all of the medication by pushing in the PLUNGER until the PLUNGER HEAD is completely between the needle guard wings. Injection of the entire prefilled syringe contents is necessary to activate the needle guard.
After injection, maintain the pressure on the PLUNGER HEAD and remove the needle from the skin. Slowly take your thumb off the PLUNGER HEAD to allow the empty syringe to move up until the entire needle is covered by the needle guard, as shown by the illustration below:
Used syringes should be placed in a puncture-resistant container.
Login To Your Free Account