FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Venofer Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Venofer is indicated for the treatment of iron deficiency anemia in patients with chronic kidney disease (CKD).
History
There is currently no drug history available for this drug.
Other Information
Venofer (iron sucrose injection, USP), an iron replacement product, is a brown, sterile, aqueous, complex of polynuclear iron (III)-hydroxide in sucrose for intravenous use. Iron sucrose injection has a molecular weight of approximately 34,000 to 60,000 daltons and a proposed structural formula:
[Na2Fe5O8(OH) ·3(H2O)]n ·m(C12H22O11)
where: n is the degree of iron polymerization and m is the number of sucrose molecules associated with the iron (III)-hydroxide.
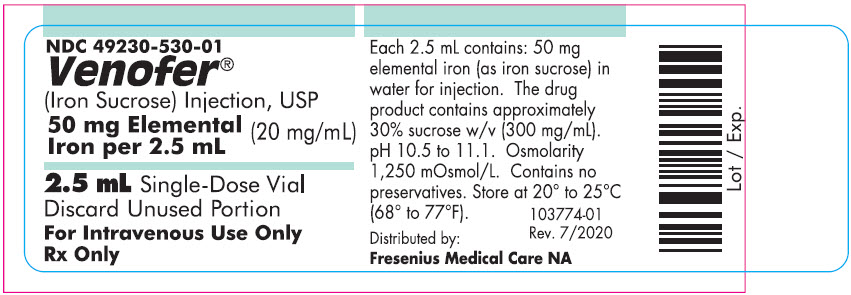
Each mL contains 20 mg elemental iron as iron sucrose in water for injection. Venofer is available in 10 mL single-use vials (200 mg elemental iron per 10 mL), 5 mL single-use vials (100 mg elemental iron per 5 mL), and 2.5 mL single-use vials (50 mg elemental iron per 2.5 mL). The drug product contains approximately 30% sucrose w/v (300 mg/mL) and has a pH of 10.5 to 11.1. The product contains no preservatives. The osmolarity of the injection is 1,250 mOsmol/L.
Sources
Venofer Manufacturers
-
Fresenius Medical Care North America
![Venofer (Iron Sucrose) Injection, Solution [Fresenius Medical Care North America]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Venofer | Fresenius Medical Care North America
![Venofer (Iron Sucrose) Injection, Solution [Fresenius Medical Care North America] Venofer (Iron Sucrose) Injection, Solution [Fresenius Medical Care North America]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Venofer must only be administered intravenously either by slow injection or by infusion. The dosage of Venofer is expressed in mg of elemental iron. Each mL contains 20 mg of elemental iron.
2.1 Adult Patients with Hemodialysis Dependent-Chronic Kidney Disease (HDD-CKD)Administer Venofer 100 mg undiluted as a slow intravenous injection over 2 to 5 minutes, or as an infusion of 100 mg diluted in a maximum of 100 mL of 0.9% NaCl over a period of at least 15 minutes, per consecutive hemodialysis session. Venofer should be administered early during the dialysis session. The usual total treatment course of Venofer is 1000 mg. Venofer treatment may be repeated if iron deficiency reoccurs.
2.2 Adult Patients with Non-Dialysis Dependent-Chronic Kidney Disease (NDD-CKD)Administer Venofer 200 mg undiluted as a slow intravenous injection over 2 to 5 minutes or as an infusion of 200 mg in a maximum of 100 mL of 0.9% NaCl over a period of 15 minutes. Administer on 5 different occasions over a 14 day period. There is limited experience with administration of an infusion of 500 mg of Venofer, diluted in a maximum of 250 mL of 0.9% NaCl, over a period of 3.5 to 4 hours on Day 1 and Day 14. Venofer treatment may be repeated if iron deficiency reoccurs.
2.3 Adult Patients with Peritoneal Dialysis Dependent-Chronic Kidney Disease (PDD-CKD)Administer Venofer in 3 divided doses, given by slow intravenous infusion, within a 28 day period: 2 infusions each of 300 mg over 1.5 hours 14 days apart followed by one 400 mg infusion over 2.5 hours 14 days later. Dilute Venofer in a maximum of 250 mL of 0.9% NaCl. Venofer treatment may be repeated if iron deficiency reoccurs.
2.4 Pediatric Patients (2 years of age and older) with HDD-CKD for iron maintenance treatmentThe dosing for iron replacement treatment in pediatric patients with HDD-CKD has not been established.
For iron maintenance treatment: Administer Venofer at a dose of 0.5 mg/kg, not to exceed 100 mg per dose, every two weeks for 12 weeks given undiluted by slow intravenous injection over 5 minutes or diluted in 25 mL of 0.9% NaCl and administered over 5 to 60 minutes. Venofer treatment may be repeated if necessary.
2.5 Pediatric Patients (2 years of age and older) with NDD-CKD or PDD-CKD who are on erythropoietin therapy for iron maintenance treatmentThe dosing for iron replacement treatment in pediatric patients with NDD-CKD or PDD-CKD has not been established.
For iron maintenance treatment: Administer Venofer at a dose of 0.5 mg/kg, not to exceed 100 mg per dose, every four weeks for 12 weeks given undiluted by slow intravenous injection over 5 minutes or diluted in 25 mL of 0.9% NaCl and administered over 5 to 60 minutes. Venofer treatment may be repeated if necessary.
-
American Regent, Inc.
![Venofer (Iron Sucrose) Injection, Solution [American Regent, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Venofer | American Regent, Inc.
![Venofer (Iron Sucrose) Injection, Solution [American Regent, Inc.] Venofer (Iron Sucrose) Injection, Solution [American Regent, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Venofer must only be administered intravenously either by slow injection or by infusion. The dosage of Venofer is expressed in mg of elemental iron. Each mL contains 20 mg of elemental iron.
2.1 Adult Patients with Hemodialysis Dependent-Chronic Kidney Disease (HDD-CKD)Administer Venofer 100 mg undiluted as a slow intravenous injection over 2 to 5 minutes, or as an infusion of 100 mg diluted in a maximum of 100 mL of 0.9% NaCl over a period of at least 15 minutes, per consecutive hemodialysis session. Venofer should be administered early during the dialysis session. The usual total treatment course of Venofer is 1000 mg. Venofer treatment may be repeated if iron deficiency reoccurs.
2.2 Adult Patients with Non-Dialysis Dependent-Chronic Kidney Disease (NDD-CKD)Administer Venofer 200 mg undiluted as a slow intravenous injection over 2 to 5 minutes or as an infusion of 200 mg in a maximum of 100 mL of 0.9% NaCl over a period of 15 minutes. Administer on 5 different occasions over a 14 day period. There is limited experience with administration of an infusion of 500 mg of Venofer, diluted in a maximum of 250 mL of 0.9% NaCl, over a period of 3.5 to 4 hours on Day 1 and Day 14. Venofer treatment may be repeated if iron deficiency reoccurs.
2.3 Adult Patients with Peritoneal Dialysis Dependent-Chronic Kidney Disease (PDD-CKD)Administer Venofer in 3 divided doses, given by slow intravenous infusion, within a 28 day period: 2 infusions each of 300 mg over 1.5 hours 14 days apart followed by one 400 mg infusion over 2.5 hours 14 days later. Dilute Venofer in a maximum of 250 mL of 0.9% NaCl. Venofer treatment may be repeated if iron deficiency reoccurs.
2.4 Pediatric Patients (2 years of age and older) with HDD-CKD for iron maintenance treatmentThe dosing for iron replacement treatment in pediatric patients with HDD-CKD has not been established.
For iron maintenance treatment: Administer Venofer at a dose of 0.5 mg/kg, not to exceed 100 mg per dose, every two weeks for 12 weeks given undiluted by slow intravenous injection over 5 minutes or diluted in 25 mL of 0.9% NaCl and administered over 5 to 60 minutes. Venofer treatment may be repeated if necessary.
2.5 Pediatric Patients (2 years of age and older) with NDD-CKD or PDD-CKD who are on erythropoietin therapy for iron maintenance treatmentThe dosing for iron replacement treatment in pediatric patients with NDD-CKD or PDD-CKD has not been established.
For iron maintenance treatment: Administer Venofer at a dose of 0.5 mg/kg, not to exceed 100 mg per dose, every four weeks for 12 weeks given undiluted by slow intravenous injection over 5 minutes or diluted in 25 mL of 0.9% NaCl and administered over 5 to 60 minutes. Venofer treatment may be repeated if necessary.
Login To Your Free Account


![Venofer (Iron Sucrose) Injection, Solution [American Regent, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=626dc9e5-c6b4-4f9c-9bf4-774fd3ae619a&name=venofer-10.jpg)