Prevalence of Diabetic Ketoacidosis Reported by Invokana Users
Top 10 Drugs That Report Diabetic Ketoacidosis as a Side Effect
New Concerns About High Levels of Blood Acid
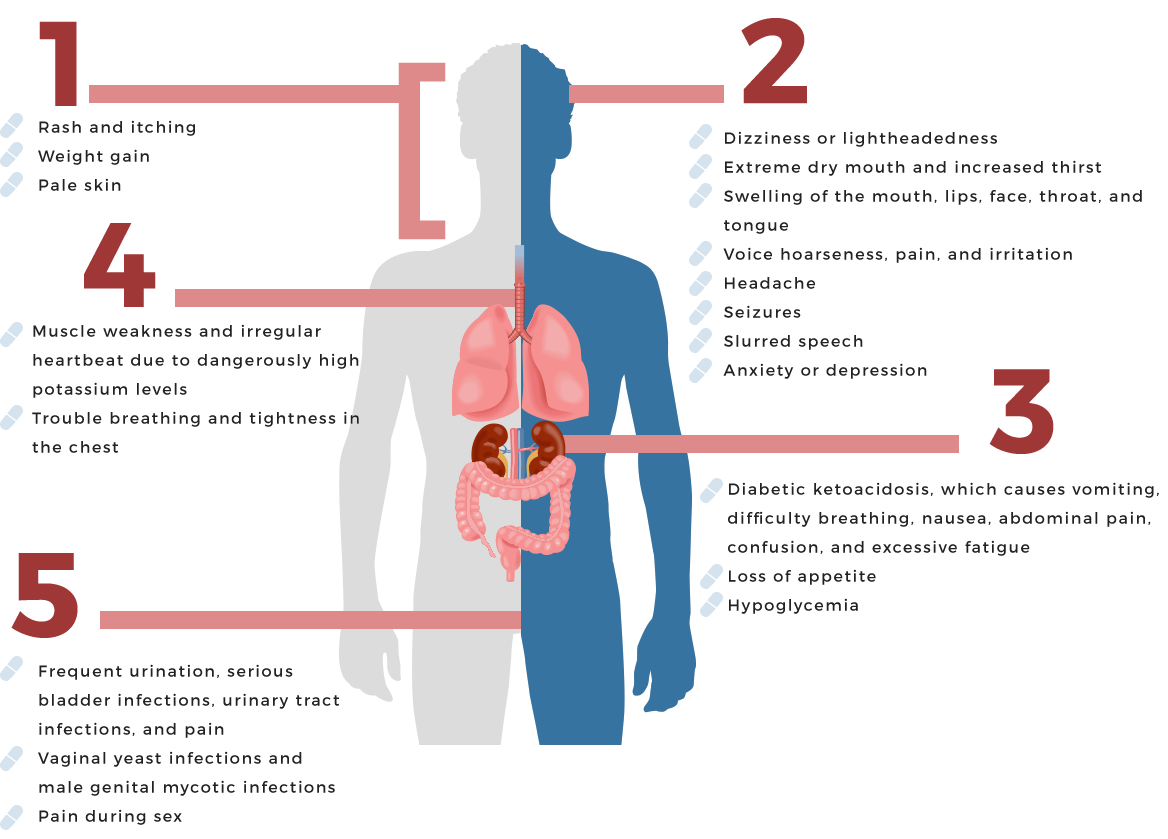
Now, only two years after its approval, the FDA has issued a warning that Invokana could cause dangerously high levels of blood acids in some patients. The agency issued the warning in May 2015, citing 20 cases of diabetic ketoacidosis, a condition caused by the body producing abnormally high levels of blood acids known as ketones. The warning stated that the FDA identified at least 20 incidents of acidosis in people taking this drug and two similar medications, Farxiga and Jardiance, between March 2013 and June 2014. The cases of acidosis involved emergency room visits or hospitalization. The information was collected by the FDA’s Adverse Event Reporting System (FAERS) database, which is designed to support post-marketing safety programs. The reports are provided on a voluntary basis by healthcare professionals and consumers either directly to the FDA or to the manufacturers of the drug. Manufacturers who receive reports are required to send them to the FDA. (Invokana)