FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
3m Avagard Foaming Instant Hand Antiseptic Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
Flammable, keep away from fire or flame. For external use only.
When using this product keep out of eyes. If contact with eyes occurs, rinse promptly and thoroughly with water.
Stop use and ask a doctor if significant irritation or sensitization develops.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions Apply to clean, dry hands. Apply sufficient amount to thoroughly wet all surfaces of hands and fingers. Rub onto hands until dry.
Other information store at 20-25°c (68-77°F)
Stop use and ask a doctor if significant irritation or sensitization develops.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Uses instant healthcare personnel hand antiseptic
- reduces bacteria that potentially can cause disease
- recommended for repeated use
History
There is currently no drug history available for this drug.
Other Information
There are no additional details available for this product.
Sources
3m Avagard Foaming Instant Hand Antiseptic Manufacturers
-
3m Health Care
![3m Avagard Foaming Instant Hand Antiseptic (Alcohol) Liquid [3m Health Care ]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
3m Avagard Foaming Instant Hand Antiseptic | Dava Pharmaceuticals, Inc.
![3m Avagard Foaming Instant Hand Antiseptic (Alcohol) Liquid [3m Health Care ] 3m Avagard Foaming Instant Hand Antiseptic (Alcohol) Liquid [3m Health Care ]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
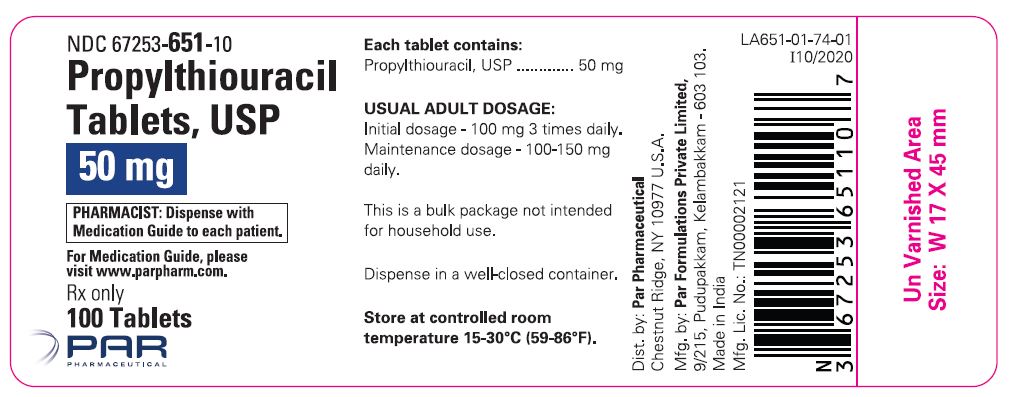
Propylthiouracil is administered orally. The total daily dosage is usually given in 3 equal doses at approximately 8 hour intervals.
AdultsThe initial dose is 300 mg daily. In patients with severe hyperthyroidism, very large goiters, or both, the initial dose may be increased to 400 mg daily; an occasional patient will require 600 to 900 mg daily initially. The usual maintenance dose is 100 to 150 mg daily.
Pediatric PatientsPropylthiouracil is generally not recommended for use in the pediatric patient population except in rare instances in which other alternative therapies are not appropriate options. Studies evaluating appropriate dosing regimen have not been conducted in the pediatric population although general practice would suggest initiation of therapy in patients 6 years or older at a dosage of 50 mg daily with careful upward titration based on clinical response and evaluation of TSH and free T4 levels. Although cases of severe liver injury have been reported with doses as low as 50 mg/day, most cases were associated with doses of 300 mg/day and higher.
Geriatric PatientsClinical studies of propylthiouracil did not include sufficient numbers of subjects aged 65 or over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
In general, dose selection for an elderly patient should be cautious reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Login To Your Free Account