FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Acd Solution Modified Solution Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
ACD solution modified is to be used in the labeling of red blood cells for intravenous administration with Cr-51 Sodium Chromate.
History
There is currently no drug history available for this drug.
Other Information
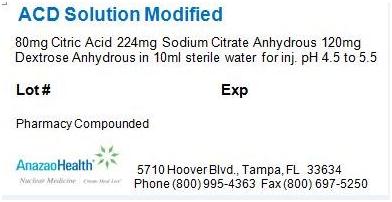
Each 100 mL vial contains 80 mg citric acid, 224 mg sodium citrate anhydrous, and 120 mg dextrose anhydrous in a sterile, non-pyrogenic solution of 10 mL. The pH of the solution has been adjusted to be between 4.5 to 5.5
Sources
Acd Solution Modified Solution Manufacturers
-
Anazaohealth Corporation
![Acd Solution Modified Solution [Anazaohealth Corporation]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Acd Solution Modified Solution | Anazaohealth Corporation
![Acd Solution Modified Solution [Anazaohealth Corporation] Acd Solution Modified Solution [Anazaohealth Corporation]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Red Blood Cell Labeling Procedure
Labeling may be performed without washing or centrifugation steps directly in the reaction vial. A 30 to 50 mL sample of whole blood is withdrawn from the patient and added aseptically to a vial of ACD Solution Modified. 50 to 150 microcuries of Sodium Chromate 51 is then injected into the reaction vial using a shielded syringe. The amount of radioactivity added to the vial willdepend on the intended use of the labeled red blood cells.
The suspension is incubated for 30 to 60 minutes at room temperature with frequent, gentle agitation. After incubation, 100 mg Ascorbic Acid Injection is injected into the vial. The ascorbic acid reduces any remaining unbound dianionic chromium 51 to the anionic state which does not penetrate red blood cells; thus in vivo labeling of red blood cells is prevented. Storage and HandlingStore the product at room temperature
Login To Your Free Account