Aggrenox Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
AGGRENOX is indicated to reduce the risk of stroke in patients who have had transient ischemia of the brain or completed ischemic stroke due to thrombosis.
History
There is currently no drug history available for this drug.
Other Information
AGGRENOX is a combination antiplatelet agent intended for oral administration. Each hard gelatin capsule contains 200 mg dipyridamole in an extended-release form and 25 mg aspirin, as an immediate-release sugar-coated tablet. In addition, each capsule contains the following inactive ingredients: acacia, aluminum stearate, colloidal silicon dioxide, corn starch, dimethicone, hypromellose, hypromellose phthalate, lactose monohydrate, methacrylic acid copolymer, microcrystalline cellulose, povidone, stearic acid, sucrose, talc, tartaric acid, titanium dioxide and triacetin.
Each capsule shell contains gelatin, red iron oxide and yellow iron oxide, titanium dioxide and water.
Dipyridamole
Dipyridamole is an antiplatelet agent chemically described as 2,2',2'',2'''-[(4,8-Dipiperidinopyrimido[5,4-d]pyrimidine-2,6-diyl)dinitrilo]-tetraethanol. It has the following structural formula:
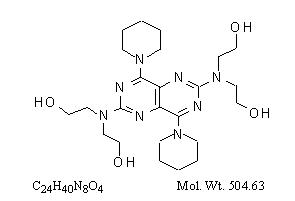
Dipyridamole is an odorless yellow crystalline substance, having a bitter taste. It is soluble in dilute acids, methanol and chloroform, and is practically insoluble in water.
Aspirin
The antiplatelet agent aspirin (acetylsalicylic acid) is chemically known as benzoic acid, 2-(acetyloxy)-, and has the following structural formula:
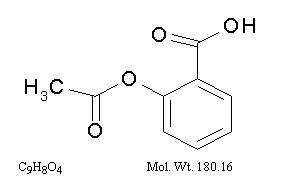
Aspirin is an odorless white needle-like crystalline or powdery substance. When exposed to moisture, aspirin hydrolyzes into salicylic and acetic acids, and gives off a vinegary odor. It is highly lipid soluble and slightly soluble in water.
Sources





![Aggrenox (Aspirin And Dipyridamole) Capsule [Physicians Total Care, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=e729d829-4bd0-4074-85ea-e0f9f5d7cdb5&name=5143.jpg)
![Aggrenox (Aspirin And Dipyridamole) Capsule [Boehringer Ingelheim Pharmaceuticals, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=938ab0b5-8377-404a-8f61-5c630bda5932&name=carton000111.jpg)
![Aggrenox (Aspirin And Dipyridamole) Capsule [Carilion Materials Management]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=f07c8535-f9fb-4697-9b63-1ab22210efc9&name=68151-3971.jpg)