Atrovent Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
Immediate hypersensitivity reactions may occur after administration of ipratropium bromide, as demonstrated by urticaria, angioedema, rash, bronchospasm, anaphylaxis, and oropharyngeal edema. If such a reaction occurs, therapy with ATROVENT Nasal Spray 0.03% should be stopped at once and alternative treatment should be considered.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
ATROVENT Nasal Spray 0.03% is indicated for the symptomatic relief of rhinorrhea associated with allergic and nonallergic perennial rhinitis in adults and children age 6 years and older. ATROVENT Nasal Spray 0.03% does not relieve nasal congestion, sneezing, or postnasal drip associated with allergic or nonallergic perennial rhinitis.
History
There is currently no drug history available for this drug.
Other Information
The active ingredient in ATROVENT Nasal Spray is ipratropium bromide (as the monohydrate). It is an anticholinergic agent chemically described as 8-azoniabicyclo[3.2.1]octane, 3-(3-hydroxy-1-oxo-2-phenylpropoxy)-8-methyl-8-(1-methylethyl)-, bromide monohydrate, (3-endo, 8-syn)-: a synthetic quaternary ammonium compound, chemically related to atropine. The structural formula is:
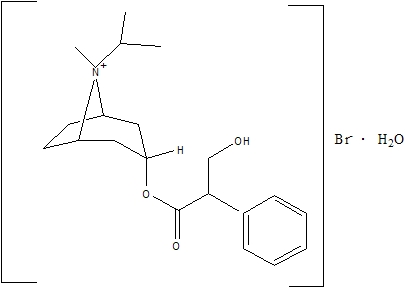
C20H30BrNO3•H2O ipratropium bromide Mol. Wt. 430.4
Ipratropium bromide is a white to off-white crystalline substance, freely soluble in water and methanol, sparingly soluble in ethanol, and insoluble in non-polar media. In aqueous solution, it exists in an ionized state as a quaternary ammonium compound.
ATROVENT Nasal Spray 0.03% is a metered-dose, manual pump spray unit which delivers 21 mcg ipratropium bromide (on an anhydrous basis) per spray (70 μL) in an isotonic aqueous solution, pH-adjusted to 4.7 with hydrochloric acid and/or sodium hydroxide (if needed). It also contains benzalkonium chloride, edetate disodium, sodium chloride, and purified water. Each bottle contains 345 sprays.
Sources