FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Berkley And Jensen Aspirin Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Zoledronic Acid Injection is indicated for the treatment of hypercalcemia of malignancy defined as an albumin-corrected calcium (cCa) of greater than or equal to 12 mg/dL [3.0 mmol/L] using the formula: cCa in mg/dL=Ca in mg/dL + 0.8 (4.0 g/dL - patient albumin [g/dL]).
Zoledronic Acid Injection is indicated for the treatment of patients with multiple myeloma and patients with documented bone metastases from solid tumors, in conjunction with standard antineoplastic therapy. Prostate cancer should have progressed after treatment with at least one hormonal therapy.
The safety and efficacy of Zoledronic Acid Injection in the treatment of hypercalcemia associated with hyperparathyroidism or with other nontumor-related conditions have not been established.
History
There is currently no drug history available for this drug.
Other Information
Zoledronic Acid Injection contains zoledronic acid, a bisphosphonic acid which is an inhibitor of osteoclastic bone resorption. Zoledronic acid is designated chemically as (1-Hydroxy-2-imidazol-1-yl-phosphonoethyl) phosphonic acid monohydrate and its structural formula is
Zoledronic acid is a white crystalline powder. Its molecular formula is C5H10N2O7P2 • H2O and its molar mass is 290.1g/mol. Zoledronic acid is highly soluble in 0.1N sodium hydroxide solution, sparingly soluble in water and 0.1N hydrochloric acid, and practically insoluble in organic solvents. The pH of a 0.7% solution of zoledronic acid in water is approximately 2.0.
Zoledronic Acid Injection is available in 5 mL vials as a sterile liquid concentrate solution for intravenous infusion.
- Each 5 mL concentrate vial contains 4.264 mg zoledronic acid monohydrate, corresponding to 4 mg zoledronic acid on an anhydrous basis, 220 mg of mannitol, USP, water for injection, and 24 mg of sodium citrate, USP.
Inactive Ingredients: mannitol, USP, as bulking agent, water for injection, and sodium citrate, USP, as buffering agent.
Sources
Berkley And Jensen Aspirin Manufacturers
-
Bjwc
![Berkley And Jensen Aspirin (Aspirin) Tablet [Bjwc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Berkley And Jensen Aspirin | Csl Behring Ag
![Berkley And Jensen Aspirin (Aspirin) Tablet [Bjwc] Berkley And Jensen Aspirin (Aspirin) Tablet [Bjwc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
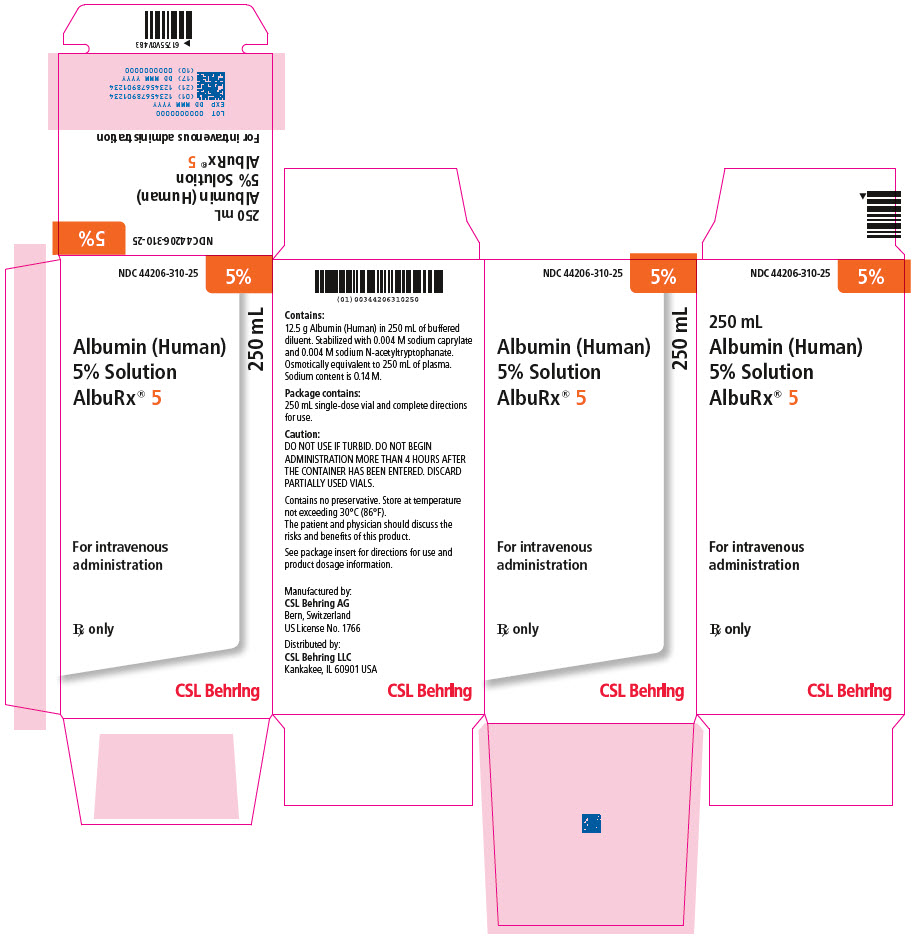
AlbuRx® 5, Albumin (Human) 5% solution must be administered intravenously. The venipuncture site should not be infected or traumatized, and should be prepared with standard aseptic technique. The solution is compatible with whole blood or packed red cells as well as the usual electrolyte and carbohydrate solutions intended for intravenous use. By contrast, it should not be mixed with protein hydrolysates, amino acid mixtures, or solutions containing alcohol. It is ready for use as contained in the bottle and may be given without regard to the blood group of the recipient.
Upon administration of AlbuRx® 5, Albumin (Human) 5% solution, there is a rapid increase of the plasma volume about equal to the volume infused. The initial dose for adults is 250 or 500 mL. The rate of infusion and the total volume administered are determined by the condition and response of the patient. A rate of 1–2 mL per minute is usually suitable in the absence of overt shock, whereas the capacity of the administration set is the only limit in the exsanguinated patient.
During resuscitation, constant monitoring of the patient provides the guidelines for treatment.
For children, a dose of 22 to 33 mL per kilogram body weight is usually adequate and close surveillance of the young patient is essential. Since patients – notably those with sepsis or severe multiple injuries – often need a circulating blood volume exceeding the prediction derived from their body weight, treatment should always be guided by the hemodynamic response and not by blood volume calculations or measurements.5
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Login To Your Free Account