FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Bexarotene Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
Bexarotene capsules induce major lipid abnormalities in most patients. These must be monitored and treated during long-term therapy. About 70% of patients with CTCL who received an initial dose of ≥ 300 mg/m2/day of Bexarotene capsules had fasting triglyceride levels greater than 2.5 times the upper limit of normal. About 55% had values over 800 mg/dL with a median of about 1200 mg/dL in those patients. Cholesterol elevations above 300 mg/dL occurred in approximately 60% and 75% of patients with CTCL who received an initial dose of 300 mg/m2/day or greater than 300 mg/m2/day, respectively. Decreases in high density lipoprotein (HDL) cholesterol to less than 25 mg/dL were seen in about 55% and 90% of patients receiving an initial dose of 300 mg/m2/day or greater than 300 mg/m2/day, respectively, of Bexarotene capsules. The effects on triglycerides, HDL cholesterol, and total cholesterol were reversible with cessation of therapy, and could generally be mitigated by dose reduction or concomitant antilipemic therapy.
Fasting blood lipid determinations should be performed before Bexarotene capsules therapy is initiated and weekly until the lipid response to Bexarotene capsules is established, which usually occurs within 2 to 4 weeks, and at 8 week intervals thereafter. Fasting triglycerides should be normal or normalized with appropriate intervention prior to initiating Bexarotene capsules therapy. Attempts should be made to maintain triglyceride levels below 400 mg/dL to reduce the risk of clinical sequelae (see WARNINGS: Pancreatitis). If fasting triglycerides are elevated or become elevated during treatment, antilipemic therapy should be instituted, and if necessary, the dose of Bexarotene capsules reduced or suspended. In the 300 mg/m2/day initial dose group, 60% of patients were given lipid lowering drugs. Atorvastatin was used in 48% (73/152) of patients with CTCL. Because of a potential drug-drug interaction (see PRECAUTIONS:Drug-Drug Interactions), gemfibrozil is not recommended for use with Bexarotene capsules.
Acute pancreatitis has been reported in four patients with CTCL and in six patients with non-CTCL cancers treated with Bexarotene capsules; the cases were associated with marked elevations of fasting serum triglycerides, the lowest being 770 mg/dL in one patient. One patient with advanced non-CTCL cancer died of pancreatitis. Patients with CTCL who have risk factors for pancreatitis (e.g., prior pancreatitis, uncontrolled hyperlipidemia, excessive alcohol consumption, uncontrolled diabetes mellitus, biliary tract disease, and medications known to increase triglyceride levels or to be associated with pancreatic toxicity) should generally not be treated with Bexarotene capsules (see WARNINGS: Lipids abnormalities and PRECAUTIONS:Laboratory Tests).
For patients with CTCL receiving an initial dose of 300 mg/m2/day of Bexarotene capsules, elevations in liver function tests (LFTs) have been observed in 5% (SGOT/AST), 2% (SGPT/ALT), and 0% (bilirubin). In contrast, with an initial dose greater than 300 mg/m2/day of Bexarotene capsules, the incidence of LFT elevations was higher at 7% (SGOT/AST), 9% (SGPT/ALT), and 6% (bilirubin). Two patients developed cholestasis, including one patient who died of liver failure.
In clinical trials, elevation of LFTs resolved within one month in 80% of patients following a decrease in dose or discontinuation of therapy. Baseline LFTs should be obtained, and LFTs should be carefully monitored after 1, 2 and 4 weeks of treatment initiation, and if stable, at least every 8 weeks thereafter during treatment. Consideration should be given to a suspension or discontinuation of Bexarotene capsules if test results reach greater than three times the upper limit of normal values for SGOT/AST, SGPT/ALT, or bilirubin.
No specific studies have been conducted with Bexarotene capsules in patients with hepatic insufficiency. Because less than 1% of the dose is excreted in the urine unchanged and there is in vitro evidence of extensive hepatic contribution to bexarotene elimination, hepatic impairment would be expected to lead to greatly decreased clearance. Bexarotene capsules should be used only with great caution in this population.
Bexarotene capsules induce biochemical evidence of or clinical hypothyroidism in about half of all patients treated, causing a reversible reduction in thyroid hormone (total thyroxine [total T4]) and thyroid-stimulating hormone (TSH) levels. The incidence of decreases in TSH and total T4 were about 60% and 45%, respectively, in patients with CTCL receiving an initial dose of 300 mg/m2/day. Hypothyroidism was reported as an adverse event in 29% of patients. Treatment with thyroid hormone supplements should be considered in patients with laboratory evidence of hypothyroidism. In the 300 mg/m2/day initial dose group, 37% of patients were treated with thyroid hormone replacement. Baseline thyroid function tests should be obtained and patients monitored during treatment.
A total of 18% of patients with CTCL receiving an initial dose of 300 mg/m2/day of Bexarotene capsules had reversible leukopenia in the range of 1000 to < 3000 WBC/mm3. Patients receiving an initial dose greater than 300 mg/m2/day of Bexarotene capsules had an incidence of leukopenia of 43%. No patient with CTCL treated with Bexarotene capsules developed leukopenia of less than 1000 WBC/mm3. The time to onset of leukopenia was generally four to eight weeks. The leukopenia observed in most patients was explained by neutropenia. In the 300 mg/m2/day initial dose group, the incidence of NCI Grade 3 and Grade 4 neutropenia, respectively, was 12% and 4%. The leukopenia and neutropenia experienced during Bexarotene capsules therapy resolved after dose reduction or discontinuation of treatment, on average within 30 days in 93% of the patients with CTCL and 82% of patients with non-CTCL cancers. Leukopenia and neutropenia were rarely associated with severe sequelae or serious adverse events. Determination of WBC with differential should be obtained at baseline and periodically during treatment.
Posterior subcapsular cataracts were observed in preclinical toxicity studies in rats and dogs administered bexarotene daily for 6 months. In 15 of 79 patients who had serial slit lamp examinations, new cataracts or worsening of previous cataracts were found. Because of the high prevalence and rate of cataract formation in older patient populations, the relationship of Bexarotene capsules and cataracts cannot be determined in the absence of an appropriate control group. Patients treated with Bexarotene capsules who experience visual difficulties should have an appropriate ophthalmologic evaluation.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Bexarotene capsules are indicated for the treatment of cutaneous manifestations of cutaneous T-cell lymphoma in patients who are refractory to at least one prior systemic therapy.
History
There is currently no drug history available for this drug.
Other Information
Bexarotene is a member of a subclass of retinoids that selectively activate retinoid X receptors (RXRs). These retinoid receptors have biologic activity distinct from that of retinoic acid receptors (RARs). Each soft gelatin capsule for oral administration contains 75 mg of bexarotene.
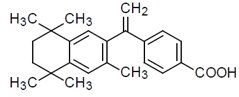
The chemical name is 4-[1-(5,6,7,8-tetrahydro-3,5,5,8,8-pentamethyl-2-naphthalenyl) ethenyl] benzoic acid, and the structural formula is as follows:
Bexarotene is an off-white to white powder with a molecular weight of 348.48 and a molecular formula of C24H28O2. It is insoluble in water and slightly soluble in vegetable oils and ethanol, USP.
Each Bexarotene capsule also contains the following inactive ingredients: polyethyelene glycol 400, NF, polysorbate 20, NF, povidone, USP and butylated hydroxyanisole, NF. The capsule shell contains gelatin NF, water, sorbitol sorbitan solution, NF, glycerin, USP and titanium dioxide, USP.
Sources
Bexarotene Manufacturers
-
Mylan Pharmaceuticals Inc.
![Bexarotene Capsule, Liquid Filled [Mylan Pharmaceuticals Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Bexarotene | Mylan Pharmaceuticals Inc.
![Bexarotene Capsule, Liquid Filled [Mylan Pharmaceuticals Inc.] Bexarotene Capsule, Liquid Filled [Mylan Pharmaceuticals Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
The recommended initial dose of Bexarotene capsules is 300 mg/m2/day. (See Table 4.) Bexarotene capsules should be taken as a single oral daily dose with a meal. See CONTRAINDICATIONS: Pregnancy: Category X section for precautions to prevent pregnancy and birth defects in women of child-bearing potential.
Table 4. Bexarotene Capsule Initial Dose Calculation According to Body Surface AreaInitial Dose Level (300 mg/m2/day)
Body Surface Area (m2)
Total Daily Dose (mg/day)
Number of 75 mg Bexarotene Capsules
0.88 - 1.12
300
4
1.13 - 1.37
375
5
1.38 - 1.62
450
6
1.63 - 1.87
525
7
1.88 - 2.12
600
8
2.13 - 2.37
675
9
2.38 - 2.62
750
10
Dose Modification GuidelinesThe 300 mg/m2/day dose level of Bexarotene capsules may be adjusted to 200 mg/m2/day then to 100 mg/m2/day, or temporarily suspended, if necessitated by toxicity. When toxicity is controlled, doses may be carefully readjusted upward. If there is no tumor response after 8 weeks of treatment and if the initial dose of 300 mg/m2/day is well tolerated, the dose may be escalated to 400 mg/m2/day with careful monitoring.
Duration of TherapyIn clinical trials in CTCL, Bexarotene capsules were administered for up to 97 weeks.
Bexarotene capsules should be continued as long as the patient is deriving benefit.
Login To Your Free Account