Catapres Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
Withdrawal
Patients should be instructed not to discontinue therapy without consulting their physician. Sudden cessation of clonidine treatment has, in some cases, resulted in symptoms such as nervousness, agitation, headache, and confusion accompanied or followed by a rapid rise in blood pressure and elevated catecholamine concentrations in the plasma. The likelihood of such reactions to discontinuation of clonidine therapy appears to be greater after administration of higher doses or continuation of concomitant beta-blocker treatment and special caution is therefore advised in these situations. Rare instances of hypertensive encephalopathy, cerebrovascular accidents and death have been reported after clonidine withdrawal. When discontinuing therapy with CATAPRES, the physician should reduce the dose gradually over 2 to 4 days to avoid withdrawal symptomatology.
An excessive rise in blood pressure following discontinuation of CATAPRES-TTS transdermal therapeutic system therapy can be reversed by administration of oral clonidine hydrochloride or by intravenous phentolamine. If therapy is to be discontinued in patients receiving a beta-blocker and clonidine concurrently, the beta-blocker should be withdrawn several days before the gradual discontinuation of CATAPRES-TTS transdermal therapeutic system.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Catapres-TTS® (clonidine) transdermal therapeutic system is indicated in the treatment of hypertension. It may be employed alone or concomitantly with other antihypertensive agents.
History
There is currently no drug history available for this drug.
Other Information
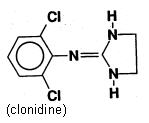
CATAPRES-TTS is a transdermal system providing continuous systemic delivery of clonidine for 7 days at an approximately constant rate. Clonidine is a centrally acting alpha-agonist hypotensive agent. It is an imidazoline derivative with the chemical name 2, 6-dichloro-N-2-imidazolidinylidenebenzenamine and has the following chemical structure:
CATAPRES
System Structure and Components
CATAPRES-TTS transdermal therapeutic system is a multi-layered film, 0.2 mm thick, containing clonidine as the active agent. The system areas are 3.5 cm2 (CATAPRES-TTS-1), 7.0 cm2 (CATAPRES-TTS-2) and 10.5 cm2 (CATAPRES-TTS-3) and the amount of drug released is directly proportional to the area (see Release Rate Concept). The composition per unit area is the same for all three doses.
Proceeding from the visible surface towards the surface attached to the skin, there are four consecutive layers: 1) a backing layer of pigmented polyester and aluminum film; 2) a drug reservoir of clonidine, mineral oil, polyisobutylene, and colloidal silicon dioxide; 3) a microporous polypropylene membrane that controls the rate of delivery of clonidine from the system to the skin surface; 4) an adhesive formulation of clonidine, mineral oil, polyisobutylene, and colloidal silicon dioxide. Prior to use, a protective slit release liner of polyester that covers the adhesive layer is removed.
Cross Section of the System:
Release Rate Concept
Catapres-TTS® (clonidine) transdermal therapeutic system is programmed to release clonidine at an approximately constant rate for 7 days. The energy for drug release is derived from the concentration gradient existing between a saturated solution of drug in the system and the much lower concentration prevailing in the skin. Clonidine flows in the direction of the lower concentration at a constant rate, limited by the rate-controlling membrane, so long as a saturated solution is maintained in the drug reservoir.
Following system application to intact skin, clonidine in the adhesive layer saturates the skin site below the system. Clonidine from the drug reservoir then begins to flow through the rate-controlling membrane and the adhesive layer of the system into the systemic circulation via the capillaries beneath the skin. Therapeutic plasma clonidine levels are achieved 2 to 3 days after initial application of CATAPRES-TTS transdermal therapeutic system.
The 3.5, 7.0, and 10.5 cm2 systems deliver 0.1, 0.2, and 0.3 mg of clonidine per day, respectively. To ensure constant release of drug for 7 days, the total drug content of the system is higher than the total amount of drug delivered. Application of a new system to a fresh skin site at weekly intervals continuously maintains therapeutic plasma concentrations of clonidine. If the CATAPRES-TTS transdermal therapeutic system is removed and not replaced with a new system, therapeutic plasma clonidine levels will persist for about 8 hours and then decline slowly over several days. Over this time period, blood pressure returns gradually to pretreatment levels.
Sources