FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Cefpodoxime Proxetil Granule Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
BEFORE THERAPY WITH CEFPODOXIME PROXETIL IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFPODOXIME, OTHER CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF CEFPODOXIME IS TO BE ADMINISTERED TO PENICILLIN SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS HYPERSENSITIVITY AMONG BETA-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO CEFPODOXIME PROXETIL OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS, INTRAVENOUS ANTIHISTAMINE, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including cefpodoxime proxetil, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
A concerted effort to monitor for C. difficile in cefpodoxime-treated patients with diarrhea was undertaken because of an increased incidence of diarrhea associated with C. difficile in early trials in normal subjects. C. difficile organisms or toxin was reported in 10% of the cefpodoxime-treated adult patients with diarrhea; however, no specific diagnosis of pseudomembranous colitis was made in these patients.
In post-marketing experience outside the United States, reports of pseudomembranous colitis associated with the use of cefpodoxime proxetil have been received.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Cefpodoxime proxetil is indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms in the conditions listed below.
Recommended dosages, durations of therapy, and applicable patient populations vary among these infections. Please see DOSAGE AND ADMINISTRATION for specific recommendations. Acute otitis media caused by Streptococcus pneumoniae (excluding penicillin-resistant strains), Streptococcus pyogenes, Haemophilus influenzae (including beta-lactamase-producing strains), or Moraxella (Branhamella) catarrhalis (including beta-lactamase producing strains).
Pharyngitis and/or tonsillitis caused by Streptococcus pyogenes.
NOTE: Only penicillin by the intramuscular route of administration has been shown to be effective in the prophylaxis of rheumatic fever. Cefpodoxime proxetil is generally effective in the eradication of streptococci from the oropharynx. However, data establishing the efficacy of cefpodoxime proxetil for the prophylaxis of subsequent rheumatic fever are not available.
Community-acquired pneumonia caused by S. pneumoniae or H. Influenzae (including beta-lactamase-producing strains).
Acute bacterial exacerbation of chronic bronchitis caused by S. pneumoniae, H. influenzae (non-beta-lactamase-producing strains only), or M. catarrhalis. Data are insufficient at this time to establish efficacy in patients with acute bacterial exacerbations of chronic bronchitis caused by beta-lactamase-producing strains of H. influenzae.
Acute, uncomplicated urethral and cervical gonorrhea caused by Neisseria gonorrhoeae (including penicillinase-producing strains).
Acute, uncomplicated ano-rectal infections in women due to Neisseria gonorrhoeae (including penicillinase-producing strains).
NOTE: The efficacy of cefpodoxime in treating male patients with rectal infections caused by N. gonorrhoeae has not been established. Data do not support the use of cefpodoxime proxetil in the treatment of pharyngeal infections due to N. gonorrhoeae in men or women.
Uncomplicated skin and skin structure infections caused by Staphylococcus aureus (including penicillinase-producing strains) or Streptococcus pyogenes. Abscesses should be surgically drained as clinically indicated.
NOTE: In clinical trials, successful treatment of uncomplicated skin and skin structure infections was dose-related. The effective therapeutic dose for skin infections was higher than those used in other recommended indications. (See DOSAGE AND ADMINISTRATION.)
Acute maxillary sinusitis caused by Haemophilus influenzae (including beta-lactamase-producing strains), Streptococcus pneumoniae, and Moraxella catarrhalis.
Uncomplicated urinary tract infections (cystitis) caused by Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, or Staphylococcus saprophyticus.
NOTE: In considering the use of cefpodoxime proxetil in the treatment of cystitis, cefpodoxime proxetil’s lower bacterial eradication rates should be weighed against the increased eradication rates and different safety profiles of some other classes of approved agents. (See CLINICAL STUDIES section.)
Appropriate specimens for bacteriological examination should be obtained in order to isolate and identify causative organisms and to determine their susceptibility to cefpodoxime. Therapy may be instituted while awaiting the results of these studies. Once these results become available, antimicrobial therapy should be adjusted accordingly.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefpodoxime proxetil and other antibacterial drugs, cefpodoxime proxetil should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
History
There is currently no drug history available for this drug.
Other Information
Cefpodoxime proxetil is an orally administered, extended spectrum, semi-synthetic antibiotic of the cephalosporin class. The chemical name is (RS)-1(isopropoxycarbonyloxy) ethyl (+)-(6R,7R)-7-[2-(2-amino-4-thiazolyl)-2-{(Z)methoxyimino}acetamido]-3-methoxymethyl-8-oxo-5-thia-1-azabicyclo [4.2.0]oct-2-ene- 2-carboxylate.
Its molecular formula is C21H27N5O9S2 and its structural formula is represented below:
The molecular weight of cefpodoxime proxetil is 557.6.
Cefpodoxime proxetil is a prodrug; its active metabolite is cefpodoxime. All doses of cefpodoxime proxetil in this insert are expressed in terms of the active cefpodoxime moiety. The drug is supplied as flavored granules for oral suspension.
Each 5 mL of cefpodoxime proxetil for oral suspension USP contains cefpodoxime proxetil USP equivalent to 50 mg or 100 mg of cefpodoxime activity after constitution and the following inactive ingredients: lactose monohydrate, corn starch, croscarmellose sodium, ferric oxide yellow, hydroxypropyl cellulose, microcrystalline cellulose and carboxymethyl cellulose sodium, colloidal silicon dioxide, citric acid anhydrous, sodium citrate, sodium benzoate, sucrose, and citron & vanille flavorings.
Sources
Cefpodoxime Proxetil Granule Manufacturers
-
Northstar Rx Llc
![Cefpodoxime Proxetil Granule, For Suspension [Northstar Rx Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Cefpodoxime Proxetil Granule | Northstar Rx Llc
![Cefpodoxime Proxetil Granule, For Suspension [Northstar Rx Llc] Cefpodoxime Proxetil Granule, For Suspension [Northstar Rx Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
(See INDICATIONS AND USAGE for indicated pathogens.)
Cefpodoxime proxetil for oral suspension may be given without regard to food. The recommended dosages, durations of treatment, and applicable patient populations are as described in the following chart:
Adults and Adolescents (age 12 years and older): Type of Infection Total Daily Dose Dose Frequency Duration Pharyngitis and/or tonsillitis
200 mg
100 mg Q 12 hours
5 to 10 days
Acute community-acquired pneumonia
400 mg
200 mg Q 12 hours
14 days
Uncomplicated gonorrhea (men and women) and rectal gonococcal infections (women)
200 mg
single dose
Skin and skin structure
800 mg
400 mg Q 12 hours
7 to 14 days
Acute maxillary sinusitis
400 mg
200 mg Q 12 hours
10 days
Uncomplicated urinary tract infection
200 mg
100 mg Q 12 hours
7 days
Infants and Pediatric Patients (age 2 months through 12 years) Type of Infection Total Daily Dose Dose Frequency Duration Acute otitis media
10 mg/kg/day
(Max 400 mg/day)
5 mg/kg Q 12 h
(Max 200 mg/dose)
5 days
Pharyngitis and/or tonsillitis
10 mg/kg/day
(Max 200 mg/day)
5 mg/kg/dose Q 12 h
(Max 100 mg/dose)
5 to 10 days
Acute maxillary sinusitis
10 mg/kg/day
(Max 400 mg/day)
5 mg/kg Q 12 hours
(Max 200 mg/dose)
10 days
Patients with Renal Dysfunction:For patients with severe renal impairment (<30 mL/min creatinine clearance), the dosing intervals should be increased to Q 24 hours. In patients maintained on hemodialysis, the dose frequency should be 3 times/week after hemodialysis.
Patients with Cirrhosis:
When only the serum creatinine level is available, the following formula (based on sex, weight, and age of the patient) may be used to estimate creatinine clearance (mL/min). For this estimate to be valid, the serum creatinine level should represent a steady state of renal function.
Males: Weight (kg) x (140 - age)
(mL/min) 72 x serum creatinine (mg/100 mL)
Females: 0.85 x above value
(mL/min)Cefpodoxime pharmacokinetics in cirrhotic patients (with or without ascites) are similar to those in healthy subjects. Dose adjustment is not necessary in this population.
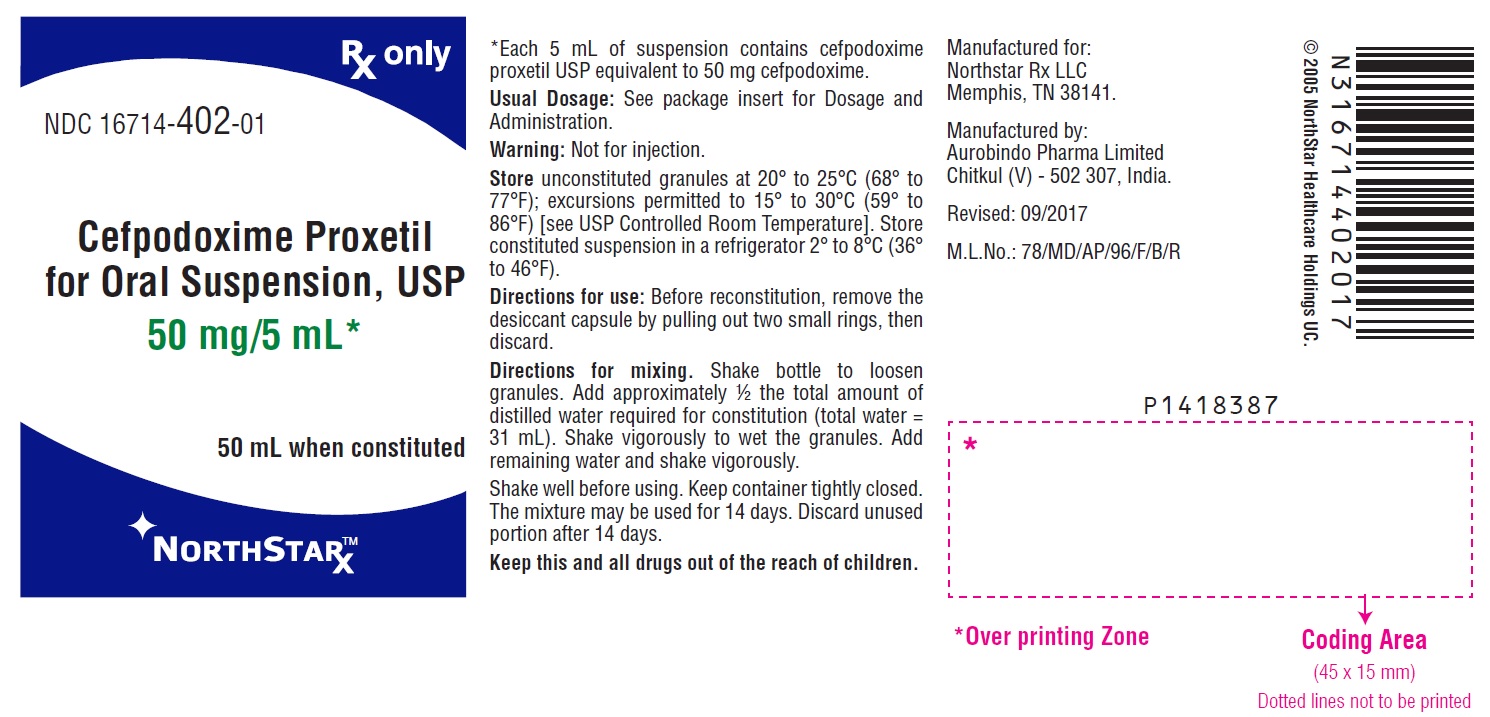
Directions for use:Before reconstitution, remove the desiccant capsule by pulling out two small rings, then throw it.
Preparation of Suspension: Constitution Directions For Oral Suspension
Constituted Volume
Final
Concentration
Directions
50 mL
50 mg per 5 mL
Suspend in a total of 31 mL of distilled water. Method:
First, shake the bottle to loosen granules. Then add the water in two approximately equal portions, shaking vigorously after each aliquot of water.
75 mL
50 mg per 5 mL
Suspend in a total of 44 mL of distilled water. Method:
First, shake the bottle to loosen granules. Then add the water in two approximately equal portions, shaking vigorously after each aliquot of water.
100 mL
50 mg per 5 mL
Suspend in a total of 57 mL of distilled water. Method:
First, shake the bottle to loosen granules. Then add the water in two approximately equal portions, shaking vigorously after each aliquot of water.
50 mL
100 mg per 5 mL
Suspend in a total of 30 mL of distilled water. Method:
First, shake the bottle to loosen granules. Then add the water in two approximately equal portions, shaking vigorously after each aliquot of water.
75 mL
100 mg per 5 mL
Suspend in a total of 43 mL of distilled water. Method:
First, shake the bottle to loosen granules. Then add the water in two approximately equal portions, shaking vigorously after each aliquot of water.
100 mL
100 mg per 5 mL
Suspend in a total of 57 mL of distilled water. Method:
First, shake the bottle to loosen granules. Then add the water in two approximately equal portions, shaking vigorously after each aliquot of water.
After mixing, the suspension should be stored in a refrigerator, 2° to 8°C (36° to 46°F). Shake well before using. Keep container tightly closed. The mixture may be used for 14 days. Discard unused portion after 14 days.
-
Sandoz Inc
![Cefpodoxime Proxetil Granule, For Suspension [Sandoz Inc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Cefpodoxime Proxetil Granule | Sandoz Inc
![Cefpodoxime Proxetil Granule, For Suspension [Sandoz Inc] Cefpodoxime Proxetil Granule, For Suspension [Sandoz Inc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
(See INDICATIONS AND USAGE for indicated pathogens.)
Granules for Oral SuspensionCefpodoxime Proxetil for Oral Suspension, USP may be given without regard to food. The recommended dosages, durations of treatment, and applicable patient population are as described in the following chart:
Adults and Adolescents (age 12 years and older) Type of Infection Total Daily Dose Dose Frequency DurationPharyngitis and/or tonsillitis
200 mg
100 mg Q 12 hours
5 to 10 days
Acute community-acquired pneumonia
400 mg
200 mg Q 12 hours
14 days
Uncomplicated gonorrhea (men and women) and rectal gonococcal infections (women)
200 mg
single dose
Skin and skin structure
800 mg
400 mg Q 12 hours
7 to 14 days
Acute maxillary sinusitis
400 mg
200 mg Q 12 hours
10 days
Uncomplicated urinary tract infection
200 mg
100 mg Q 12 hours
7 days
Infants and Pediatric Patients (age 2 months through 12 years) Type of Infection Total Daily Dose Dose Frequency DurationAcute otitis media
10 mg/kg/day
(Max 400 mg/day)5 mg/kg Q 12 h
(Max 200 mg/dose)5 days
Pharyngitis and/or tonsillitis
10 mg/kg/day
(Max 200 mg/day)5 mg/kg/dose Q 12 h
(Max 100 mg/dose)5 to 10 days
Acute maxillary sinusitis
10 mg/kg/day
(Max 400 mg/day)5 mg/kg Q 12 hours
(Max 200 mg/dose)10 days
Patients with Renal DysfunctionFor patients with severe renal impairment (<30 mL/min creatinine clearance), the dosing intervals should be increased to Q 24 hours. In patients maintained on hemodialysis, the dose frequency should be 3 times/week after hemodialysis.
When only the serum creatinine level is available, the following formula (based on sex, weight, and age of the patient) may be used to estimate creatinine clearance (mL/min). For this estimate to be valid, the serum creatinine level should represent a steady state of renal function.
Males:
(mL/min)Weight (kg) × (140 - age)
72 × serum creatinine (mg/100 mL)Females:
(mL/min)0.85 × above value
Patients with CirrhosisCefpodoxime pharmacokinetics in cirrhotic patients (with or without ascites) are similar to those in healthy subjects. Dose adjustment is not necessary in this population.
Preparation of Suspension Constitution Directions for Oral Suspension Constituted
Volume Final
Concentrations
Directions50 mL
50 mg per 5 mL
Suspend in a total of 32 mL of distilled water. Method: First, shake the bottle to loosen the granules. Then add the water in two approximately equal portions, shaking vigorously after each aliquot of water.
100 mL
50 mg per 5 mL
Suspend in a total of 63 mL of distilled water. Method: First, shake the bottle to loosen the granules. Then add the water in two approximately equal portions, shaking vigorously after each aliquot of water.
50 mL
100 mg per 5 mL
Suspend in a total of 32 mL of distilled water. Method: First, shake the bottle to loosen the granules. Then add the water in two approximately equal portions, shaking vigorously after each aliquot of water.
100 mL
100 mg per 5 mL
Suspend in a total of 63 mL of distilled water. Method: First, shake the bottle to loosen the granules. Then add the water in two approximately equal portions, shaking vigorously after each aliquot of water.
After mixing, the suspension should be stored in a refrigerator, 2° to 8°C (36° to 46°F). Shake well before using. Keep container tightly closed. The mixture may be used for 14 days. Discard unused portion after 14 days.
-
Aurobindo Pharma Limited
![Cefpodoxime Proxetil Granule, For Suspension [Aurobindo Pharma Limited]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Cefpodoxime Proxetil Granule | Aurobindo Pharma Limited
![Cefpodoxime Proxetil Granule, For Suspension [Aurobindo Pharma Limited] Cefpodoxime Proxetil Granule, For Suspension [Aurobindo Pharma Limited]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
(See INDICATIONS AND USAGE for indicated pathogens.)
Cefpodoxime proxetil for oral suspension may be given without regard to food. The recommended dosages, durations of treatment, and applicable patient populations are as described in the following chart:
Adults and Adolescents (age 12 years and older): Type of Infection Total Daily Dose Dose Frequency Duration Pharyngitis and/or tonsillitis
200 mg
100 mg Q 12 hours
5 to 10 days
Acute community-acquired pneumonia
400 mg
200 mg Q 12 hours
14 days
Uncomplicated gonorrhea (men and women) and rectal gonococcal infections (women)
200 mg
single dose
Skin and skin structure
800 mg
400 mg Q 12 hours
7 to 14 days
Acute maxillary sinusitis
400 mg
200 mg Q 12 hours
10 days
Uncomplicated urinary tract infection
200 mg
100 mg Q 12 hours
7 days
Infants and Pediatric Patients (age 2 months through 12 years): Type of Infection Total Daily Dose Dose Frequency Duration Acute otitis media
10 mg/kg/day
(Max 400 mg/day)
5 mg/kg Q 12 h
(Max 200 mg/dose)
5 days
Pharyngitis and/or tonsillitis
10 mg/kg/day
(Max 200 mg/day)
5 mg/kg/dose Q 12 h
(Max 100 mg/dose)
5 to 10 days
Acute maxillary sinusitis
10 mg/kg/day
(Max 400 mg/day)
5 mg/kg Q 12 hours
(Max 200 mg/dose)
10 days
Patients with Renal Dysfunction:For patients with severe renal impairment (<30 mL/min creatinine clearance), the dosing intervals should be increased to Q 24 hours. In patients maintained on hemodialysis, the dose frequency should be 3 times/week after hemodialysis.
Patients with Cirrhosis:
When only the serum creatinine level is available, the following formula (based on sex, weight, and age of the patient) may be used to estimate creatinine clearance (mL/min). For this estimate to be valid, the serum creatinine level should represent a steady state of renal function.
Males: Weight (kg) x (140 - age)
(mL/min) 72 x serum creatinine (mg/100 mL)
Females: 0.85 x above value
(mL/min)Cefpodoxime pharmacokinetics in cirrhotic patients (with or without ascites) are similar to those in healthy subjects. Dose adjustment is not necessary in this population.
Directions for use:Before reconstitution, remove the desiccant capsule by pulling out two small rings, then throw it.
Preparation of Suspension: Constitution Directions For Oral Suspension
Constituted Volume
Final
Concentration
Directions
50 mL
50 mg per 5 mL
Suspend in a total of 31 mL of distilled water. Method:
First, shake the bottle to loosen granules. Then add the water in two approximately equal portions, shaking vigorously after each aliquot of water.
75 mL
50 mg per 5 mL
Suspend in a total of 44 mL of distilled water. Method:
First, shake the bottle to loosen granules. Then add the water in two approximately equal portions, shaking vigorously after each aliquot of water.
100 mL
50 mg per 5 mL
Suspend in a total of 57 mL of distilled water. Method:
First, shake the bottle to loosen granules. Then add the water in two approximately equal portions, shaking vigorously after each aliquot of water.
50 mL
100 mg per 5 mL
Suspend in a total of 30 mL of distilled water. Method:
First, shake the bottle to loosen granules. Then add the water in two approximately equal portions, shaking vigorously after each aliquot of water.
75 mL
100 mg per 5 mL
Suspend in a total of 43 mL of distilled water. Method:
First, shake the bottle to loosen granules. Then add the water in two approximately equal portions, shaking vigorously after each aliquot of water.
100 mL
100 mg per 5 mL
Suspend in a total of 57 mL of distilled water. Method:
First, shake the bottle to loosen granules. Then add the water in two approximately equal portions, shaking vigorously after each aliquot of water.
After mixing, the suspension should be stored in a refrigerator, 2° to 8°C (36° to 46°F). Shake well before using. Keep container tightly closed. The mixture may be used for 14 days. Discard unused portion after 14 days.
Login To Your Free Account


![Cefpodoxime Proxetil Granule, For Suspension [Sandoz Inc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=06a35453-8356-4690-aacb-3845cdc84102&name=75ef1f0b-f03b-49e3-aeaf-209e8848ddd3-02.jpg)
![Cefpodoxime Proxetil Granule, For Suspension [Aurobindo Pharma Limited]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=63777889-d306-4fc3-88da-b24859040ef4&name=cefpodoxime-fig1.jpg)