FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Childrens Sabadil Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Oxycodone hydrochloride oral solution USP, 100 mg per 5 mL (20 mg per mL) an opioid agonist is indicated for the relief of moderate to severe acute and chronic pain in opioid-tolerant patients.
Oxycodone hydrochloride oral solution USP, 100 mg per 5 mL (20 mg per mL) may cause fatal respiratory depression when administered to patients not previously exposed to opioids. Patients considered to be opioid tolerant are those who are taking at least 30 mg of oral oxycodone per day, or at least 60 mg oral morphine per day, or at least 12 mg hydromorphone per day, or an equianalgesic dose of another opioid, for a week or longer.
History
There is currently no drug history available for this drug.
Other Information
Oxycodone hydrochloride is a white to off-white, fine crystalline powder derived from the opium alkaloid, thebaine. It is soluble in water and slightly soluble in alcohol.
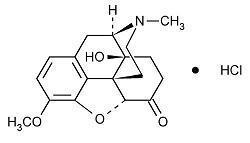
Chemically, oxycodone hydrochloride is (5R,9R,13S,14S)-4, 5α-epoxy-14-hydroxy-3-methoxy-17-methylmorphinan-6-one hydrochloride with a molecular mass of 351.82.
Oxycodone Hydrochloride Oral Solution, USP 100 mg per 5 mL (20 mg per mL): Each 1 mL of oral yellow solution contains 20 mg of oxycodone hydrochloride, USP and the following inactive ingredients: citric acid anhydrous, D&C Yellow #10, natural/artificial mixed berry flavor, purified water, sodium citrate dihydrate, sodium benzoate, saccharin sodium, sorbitol solution.
Sources
Childrens Sabadil Manufacturers
-
Laboratoires Boiron
![Childrens Sabadil (Onion, Ambrosia Artemisiifolia, Histamine Dihydrochloride, Schoenocaulon Officinale Seed, Euphrasia Stricta, Solidago Virgaurea Flowering Top) Pellet [Laboratoires Boiron]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Childrens Sabadil | Novel Laboratories, Inc.
![Childrens Sabadil (Onion, Ambrosia Artemisiifolia, Histamine Dihydrochloride, Schoenocaulon Officinale Seed, Euphrasia Stricta, Solidago Virgaurea Flowering Top) Pellet [Laboratoires Boiron] Childrens Sabadil (Onion, Ambrosia Artemisiifolia, Histamine Dihydrochloride, Schoenocaulon Officinale Seed, Euphrasia Stricta, Solidago Virgaurea Flowering Top) Pellet [Laboratoires Boiron]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Oxycodone Hydrochloride Oral Solution is available in one concentration: 100 mg per 5 mL (20 mg per mL).Take care when prescribing and administering Oxycodone Hydrochloride Oral Solution to avoid dosing errors due to confusion between mg and mL, which could result in accidental overdose and death. Take care to ensure the proper dose is communicated and dispensed. When writing prescriptions, include both the total dose in mg and the total dose in volume. Always use the enclosed calibrated oral syringe when administering Oxycodone Hydrochloride Oral Solution, 100 mg per 5 mL (20 mg per mL) to ensure the dose is measured and administered accurately.
Selection of patients for treatment with oxycodone hydrochloride should be governed by the same principles that apply to the use of similar opioid analgesics. Individualize treatment in every case, using non-opioid analgesics, opioids on an as needed basis and/or combination products, and chronic opioid therapy in a progressive plan of pain management such as outlined by the World Health Organization, the Agency for Healthcare Research and Quality, and the American Pain Society.
2.1 Individualization of DosageAs with any opioid drug product, adjust the dosing regimen for each patient individually, taking into account the patient’s prior analgesic treatment experience. In the selection of the initial dose of oxycodone hydrochloride, give attention to the following:
the total daily dose, potency and specific characteristics of the opioid the patient has been taking previously; the reliability of the relative potency estimate used to calculate the equivalent oxycodone hydrochloride dose needed; the patient’s degree of opioid tolerance; the general condition and medical status of the patient; concurrent medications; the type and severity of the patient’s pain; risk factors for abuse, addiction or diversion, including a prior history of abuse, addiction or diversion.The following dosing recommendations, therefore, can only be considered suggested approaches to what is actually a series of clinical decisions over time in the management of the pain of each individual patient.
Continual re-evaluation of the patient receiving oxycodone hydrochloride is important, with special attention to the maintenance of pain control and the relative incidence of side effects associated with therapy. During chronic therapy, especially for non-cancer-related pain, periodically re-assess the continued need for the use of opioid analgesics.
During periods of changing analgesic requirements, including initial titration, frequent contact is recommended between physician, other members of the healthcare team, the patient, and the caregiver/family.
2.2 Initiation of Therapy in Opioid Naïve PatientsStart patients who have not been receiving opioid analgesics on oxycodone hydrochloride in the following dosing range using oral solution, 5 mg per 5 mL strength. Oxycodone HCl Oral Solution: 5 to15 mg every 4 to 6 hours as needed for pain.
Titrate the dose based upon the individual patient's response to their initial dose of oxycodone hydrochloride. Adjust the dose to an acceptable level of analgesia taking into account the improvement in pain intensity and tolerability of the oxycodone by the patient.
The 100 mg per 5 mL (20 mg per mL) oral solution formulation is for use in opioid-tolerant patients only who have already been receiving opioid therapy. Use this strength only for patients that have already been titrated to a stable analgesic regimen using lower strengths of oxycodone hydrochloride and who can benefit from use of a smaller volume of oral solution.
Instruct patients how to measure and take the correct dose of Oxycodone Hydrochloride Oral Solution. Advise patients to always use the enclosed oral syringe when administering Oxycodone Hydrochloride Oral Solution 100 mg per 5 mL (20 mg per mL) to ensure the dose is measured and administered accurately. 2.3 Conversion to Oral Oxycodone HydrochlorideThere is inter-patient variability in the potency of opioid drugs and opioid formulations. Therefore, a conservative approach is advised when determining the total daily dose of Oxycodone Hydrochloride. It is better to underestimate a patient’s 24-hour oral Oxycodone Hydrochloride dose and make available rescue medication than to overestimate the 24-hour oral Oxycodone Hydrochloride dose and manage an adverse experience of overdose.
Consider the following general points regarding opioid conversions.
Conversion From Non-Oxycodone Opioids to Oral Oxycodone Hydrochloride.
In converting patients from other opioids to oxycodone hydrochloride, close observation and adjustment of dosage based upon the patient’s response to oxycodone hydrochloride is imperative. Physicians and other healthcare professionals are advised to refer to published relative potency information, keeping in mind that conversion ratios are only approximate.
Conversion From Controlled-Release Oral Oxycodone to Oral Oxycodone Hydrochloride.
The relative bioavailability of Oxycodone Hydrochloride Oral Solution compared to controlled-release oxycodone is unknown. The extended duration of release of oxycodone hydrochloride from controlled-release tablets results in reduced maximum and increased minimum plasma oxycodone hydrochloride concentrations than with shorter acting oxycodone hydrochloride products. Conversion from controlled-release tablets could lead to excessive sedation at peak serum levels. Therefore, dosage adjustment with close observation is necessary.
Conversion From Oral Oxycodone Hydrochloride to Controlled-Release Oral Oxycodone
The relative bioavailability of Oxycodone Hydrochloride Oral Solution compared to controlled-release oxycodone is unknown, so conversion to controlled-release tablets must be accompanied by close observation for signs of excessive sedation.
2.4 Maintenance of TherapyContinual re-evaluation of the patient receiving oxycodone hydrochloride is important, with special attention to the maintenance of pain management and the relative incidence of side effects associated with therapy. If the level of pain increases, effort should be made to identify the source of increased pain, while adjusting the dose as described above to decrease the level of pain. During chronic therapy, especially for non-cancer-related pain (or pain associated with other terminal illnesses), periodically reassess the continued need for the use of opioid analgesics.
2.5 Cessation of TherapyWhen a patient no longer requires therapy with oxycodone hydrochloride gradually taper the dose to prevent signs and symptoms of withdrawal in the physically dependent patient.
Login To Your Free Account