FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Ciclodan Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
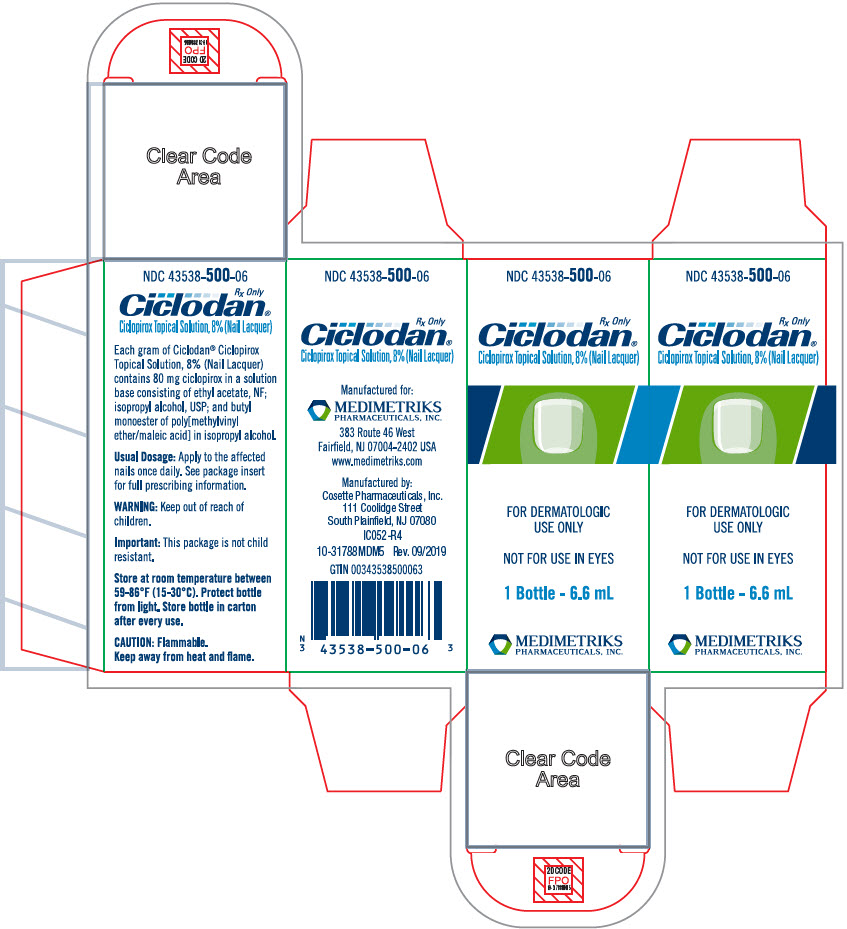
Ciclopirox Topical Solution, 8%, (Nail Lacquer), is not for ophthalmic, oral, or intravaginal use. For use on nails and immediately adjacent skin only.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
(To understand fully the indication for this product, please read the entire INDICATIONS AND USAGE section of the labeling.)
Ciclodan™ Ciclopirox Topical Solution, 8%, (Nail Lacquer), as a component of a comprehensive management program, is indicated as topical treatment in immunocompetent patients with mild to moderate onychomycosis of fingernails and toenails without lunula involvement, due to Trichophyton rubrum. The comprehensive management program includes removal of the unattached, infected nails as frequently as monthly, by a health care professional who has special competence in the diagnosis and treatment of nail disorders, including minor nail procedures.
- No studies have been conducted to determine whether ciclopirox might reduce the effectiveness of systemic antifungal agents for onychomycosis. Therefore, the concomitant use of 8% ciclopirox topical solution and systemic antifungal agents for onychomycosis, is not recommended.
- Ciclopirox Topical Solution, 8%, (Nail Lacquer), should be used only under medical supervision as described above.
- The effectiveness and safety of Ciclopirox Topical Solution, 8%, (Nail Lacquer), in the following populations has not been studied. The clinical trials with use of Ciclopirox Topical Solution, 8%, (Nail Lacquer), excluded patients who: were pregnant or nursing, planned to become pregnant, had a history of immunosuppression (e.g., extensive, persistent, or unusual distribution of dermatomycoses, extensive seborrheic dermatitis, recent or recurring herpes zoster, or persistent herpes simplex), were HIV seropositive, received organ transplant, required medication to control epilepsy, were insulin dependent diabetics or had diabetic neuropathy. Patients with severe plantar (moccasin) tinea pedis were also excluded.
- The safety and efficacy of using Ciclopirox Topical Solution, 8%, (Nail Lacquer), daily for greater than 48 weeks have not been established.
The results of use of Ciclopirox Topical Solution, 8%, (Nail Lacquer), in treatment of onychomycosis of the toenail without lunula involvement were obtained from two double-blind, placebo-controlled studies conducted in the US. In these studies, patients with onychomycosis of the great toenails without lunula involvement were treated with ciclopirox topical solution, 8% in conjunction with monthly removal of the unattached, infected toenail by the investigator. Ciclopirox Topical Solution, 8%, (Nail Lacquer), was applied for 48 weeks. At baseline, patients had 20–65% involvement of the target great toenail plate. Statistical significance was demonstrated in one of two studies for the endpoint "complete cure" (clear nail and negative mycology), and in two studies for the endpoint "almost clear" (≤10% nail involvement and negative mycology) at the end of study. These results are presented below.
| Study 312 | Study 313 | |||
|---|---|---|---|---|
| Active | Vehicle | Active | Vehicle | |
|
||||
| Complete Cure* | 6/110 (5.5%) |
1/109 (0.9%) |
10/118 (8.5%) |
0/117 (0%) |
| Almost Clear† | 7/107 (6.5%) |
1/108 (0.9%) |
14/116 (12%) |
1/115 (0.9%) |
| Negative Mycology Alone‡ | 30/105 (29%) |
12/106 (11%) |
41/115 (36%) |
10/114 (9%) |
The summary of reported patient outcomes for the ITT population at 12 weeks following the end of treatment are presented below. Note that post-treatment efficacy assessments were scheduled only for patients who achieved a complete cure.
| Study 312 | Study 313 | |||
|---|---|---|---|---|
| Active | Vehicle | Active | Vehicle | |
|
||||
| Number of Treated Patients | 112 | 111 | 119 | 118 |
| Complete Cure at Week 48 | 6 | 1 | 10 | 0 |
| Post-treatment Week 12 Outcomes: | ||||
| Patients Missing All Week 12 Assessments | 2 | 0 | 2 | 0 |
| Patients with Week 12 Assessments | 4 | 1 | 8 | 0 |
| Complete Cure | 3 | 1 | 4 | 0 |
| Almost Clear | 2* | 1 | 1* | 0 |
| Negative Mycology | 3 | 1 | 5 | 0 |
History
There is currently no drug history available for this drug.
Other Information
Ciclodan™ Ciclopirox Topical Solution, 8%, (Nail Lacquer) contains a synthetic antifungal agent, ciclopirox. It is intended for topical use on fingernails and toenails and immediately adjacent skin.
Each gram of Ciclodan™ Ciclopirox Topical Solution, 8%, (Nail Lacquer), contains 80 mg ciclopirox in a solution base consisting of ethyl acetate, NF; isopropyl alcohol, USP; and butyl monoester of poly[methylvinyl ether/maleic acid] in isopropyl alcohol. Ethyl acetate and isopropyl alcohol are solvents that vaporize after application.
Ciclodan™ Ciclopirox Topical Solution, 8%, (Nail Lacquer), is a clear, colorless to slightly yellowish solution.
The chemical name for ciclopirox is 6-cyclohexyl-1-hydroxy-4-methyl-2(1H)-pyridone, with the molecular formula C12H17NO2 and a molecular weight of 207.27. The CAS Registry Number is [29342-05-0]. The chemical structure is:

Sources
Ciclodan Manufacturers
-
Medimetriks Pharmaceuticals, Inc.
![Ciclodan (Ciclopirox) Solution [Medimetriks Pharmaceuticals, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Ciclodan | Medimetriks Pharmaceuticals, Inc.
![Ciclodan (Ciclopirox) Solution [Medimetriks Pharmaceuticals, Inc.] Ciclodan (Ciclopirox) Solution [Medimetriks Pharmaceuticals, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Ciclopirox Topical Solution, 8%, (Nail Lacquer), should be used as a component of a comprehensive management program for onychomycosis. Removal of the unattached, infected nail, as frequently as monthly, by a health care professional, weekly trimming by the patient, and daily application of the medication are all integral parts of this therapy. Careful consideration of the appropriate nail management program should be given to patients with diabetes (see PRECAUTIONS).
Nail Care By Health Care ProfessionalsRemoval of the unattached, infected nail, as frequently as monthly, trimming of onycholytic nail, and filing of excess horny material should be performed by professionals trained in treatment of nail disorders.
Nail Care By PatientPatients should file away (with emery board) loose nail material and trim nails, as required, or as directed by the health care professional, every seven days after Ciclopirox Topical Solution, 8%, (Nail Lacquer), is removed with alcohol.
Ciclopirox Topical Solution, 8%, (Nail Lacquer), should be applied once daily (preferably at bedtime or eight hours before washing) to all affected nails with the applicator brush provided. The Ciclopirox Topical Solution, 8%, (Nail Lacquer), should be applied evenly over the entire nail plate.
If possible, Ciclopirox Topical Solution, 8%, (Nail Lacquer), should be applied to the nail bed, hyponychium, and the under surface of the nail plate when it is free of the nail bed (e.g., onycholysis).
The Ciclopirox Topical Solution, 8%, (Nail Lacquer), should not be removed on a daily basis. Daily applications should be made over the previous coat and removed with alcohol every seven days. This cycle should be repeated throughout the duration of therapy.
-
Medimetriks Pharmaceuticals, Inc.
![Ciclodan (Ciclopirox Olamine) Cream Ciclodan (Ciclopirox Olamine) Kit [Medimetriks Pharmaceuticals, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Ciclodan | Medimetriks Pharmaceuticals, Inc.
![Ciclodan (Ciclopirox Olamine) Cream Ciclodan (Ciclopirox Olamine) Kit [Medimetriks Pharmaceuticals, Inc.] Ciclodan (Ciclopirox Olamine) Cream Ciclodan (Ciclopirox Olamine) Kit [Medimetriks Pharmaceuticals, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Gently massage Ciclodan® Cream into the affected and surrounding skin areas twice daily, in the morning and evening. Clinical improvement with relief of pruritus and other symptoms usually occurs within the first week of treatment. If a patient shows no clinical improvement after four weeks of treatment with Ciclodan® Cream the diagnosis should be redetermined. Patients with tinea versicolor usually exhibit clinical and mycological clearing after two weeks of treatment.
Login To Your Free Account


![Ciclodan (Ciclopirox Olamine) Cream Ciclodan (Ciclopirox Olamine) Kit [Medimetriks Pharmaceuticals, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=346422b8-003d-4c4f-8a92-1e9146c13732&name=ciclodan-02.jpg)