Ciprodex Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
FOR OTIC USE ONLY
(This product is not approved for ophthalmic use.)
NOT FOR INJECTION
CIPRODEX® Otic should be discontinued at the first appearance of a skin rash or any other sign of hypersensitivity. Serious and occasionally fatal hypersensitivity (anaphylactic) reactions, some following the first dose, have been reported in patients receiving systemic quinolones. Serious acute hypersensitivity reactions may require immediate emergency treatment.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
CIPRODEX® Otic is indicated for the treatment of infections caused by susceptible isolates of the designated microorganisms in the specific conditions listed below:
Acute Otitis Media in pediatric patients (age 6 months and older) with tympanostomy tubes due to Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Pseudomonas aeruginosa.
Acute Otitis Externa in pediatric (age 6 months and older), adult and elderly patients due to Staphylococcus aureus and Pseudomonas aeruginosa.
History
There is currently no drug history available for this drug.
Other Information
CIPRODEX® (ciprofloxacin 0.3% and dexamethasone 0.1%) Sterile Otic Suspension contains the synthetic broad-spectrum antibacterial agent, ciprofloxacin hydrochloride, combined with the anti-inflammatory corticosteroid, dexamethasone, in a sterile, preserved suspension for otic use. Each mL of CIPRODEX® Otic contains ciprofloxacin hydrochloride (equivalent to 3 mg ciprofloxacin base), 1 mg dexamethasone, and 0.1 mg benzalkonium chloride as a preservative. The inactive ingredients are boric acid, sodium chloride, hydroxyethyl cellulose, tyloxapol, acetic acid, sodium acetate, edetate disodium, and purified water. Sodium hydroxide or hydrochloric acid may be added for adjustment of pH.
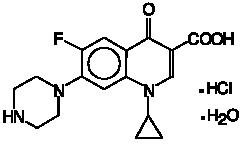
Ciprofloxacin, a fluoroquinolone is available as the monohydrochloride monohydrate salt of 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid. The empirical formula is C17H18FN3O3·HCl·H2O and the structural formula is:
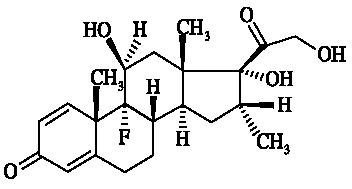
Dexamethasone, 9-fluoro-11(beta),17,21-trihydroxy-16(alpha)-methylpregna-1,4-diene-3,20-dione, is an anti-inflammatory corticosteroid. The empirical formula is C22H29FO5 and the structural formula is:
Sources





![Ciprodex (Ciprofloxacin And Dexamethasone) Suspension [Alcon, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=0ed518de-4ae1-43d1-84ff-26872d9e6a0f&name=carton.jpg)
![Ciprodex (Ciprofloxacin And Dexamethasone) Suspension [Rebel Distributors Corp]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=895c2307-aeeb-49fd-ac3d-750a463fc3ef&name=895c2307-aeeb-49fd-ac3d-750a463fc3ef-10.jpg)
![Ciprodex (Ciprofloxacin And Dexamethasone) Suspension [Clinical Solutions Wholesale]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=1c4db12f-285b-4714-9b1c-26bcdc39cebf&name=1c4db12f-285b-4714-9b1c-26bcdc39cebf-10.jpg)