FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Cobrazol Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
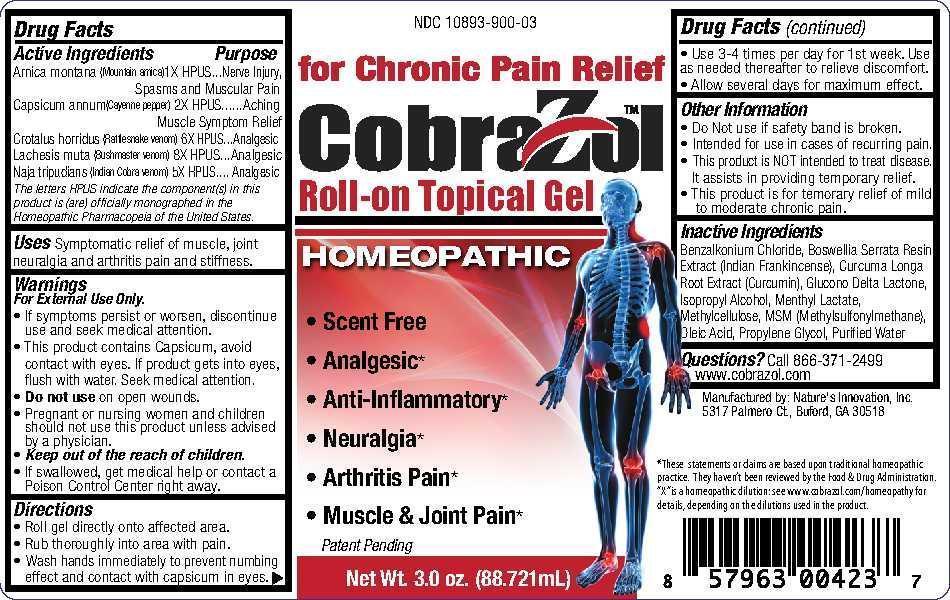
Warnings
For External Use Only.
- If symptoms persist or worsen, discontinue use and seek medical attention.
- This product contains Capsicum, avoid contact with eyes. If product gets into eyes, flushy with water. Seek medical attention.
- Do not use on open wounds.
- Pregnant or nursing women and children should not use this product unless advised by a physician.
- If swallowed, get medical help or contact a Poison Control Center right away.
- If symptoms persist or worsen, discontinue use and seek medical attention.
- Do not use on open wounds.
- If swallowed, get medical help or contact a Poison Control Center right away.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
UsesSymptomatic relief of muscle, joint neuralgia and arthritis pain and stiffness.
History
There is currently no drug history available for this drug.
Other Information
There are no additional details available for this product.
Sources
Cobrazol Manufacturers
-
Nature’s Innovation, Inc.
![Cobrazol (Arnica Montana, Capsicum Annum, Crotalus Horridus, Lachesis Muta, Naja Tripudians) Gel [Nature’s Innovation, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Cobrazol | Nature's Innovation, Inc.
![Cobrazol (Arnica Montana, Capsicum Annum, Crotalus Horridus, Lachesis Muta, Naja Tripudians) Gel [Nature’s Innovation, Inc.] Cobrazol (Arnica Montana, Capsicum Annum, Crotalus Horridus, Lachesis Muta, Naja Tripudians) Gel [Nature’s Innovation, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Directions
Roll gel directly onto affected area. Rub thoroughly into area with pain. Wash hands immediately to prevent numbing effect and contact with capsicum in eyes. Use 3-4 times per day for 1st week. Use as needed thereafter to relieve discomfort. Allow several days for maximum effect.
Login To Your Free Account