FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Combination Product Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
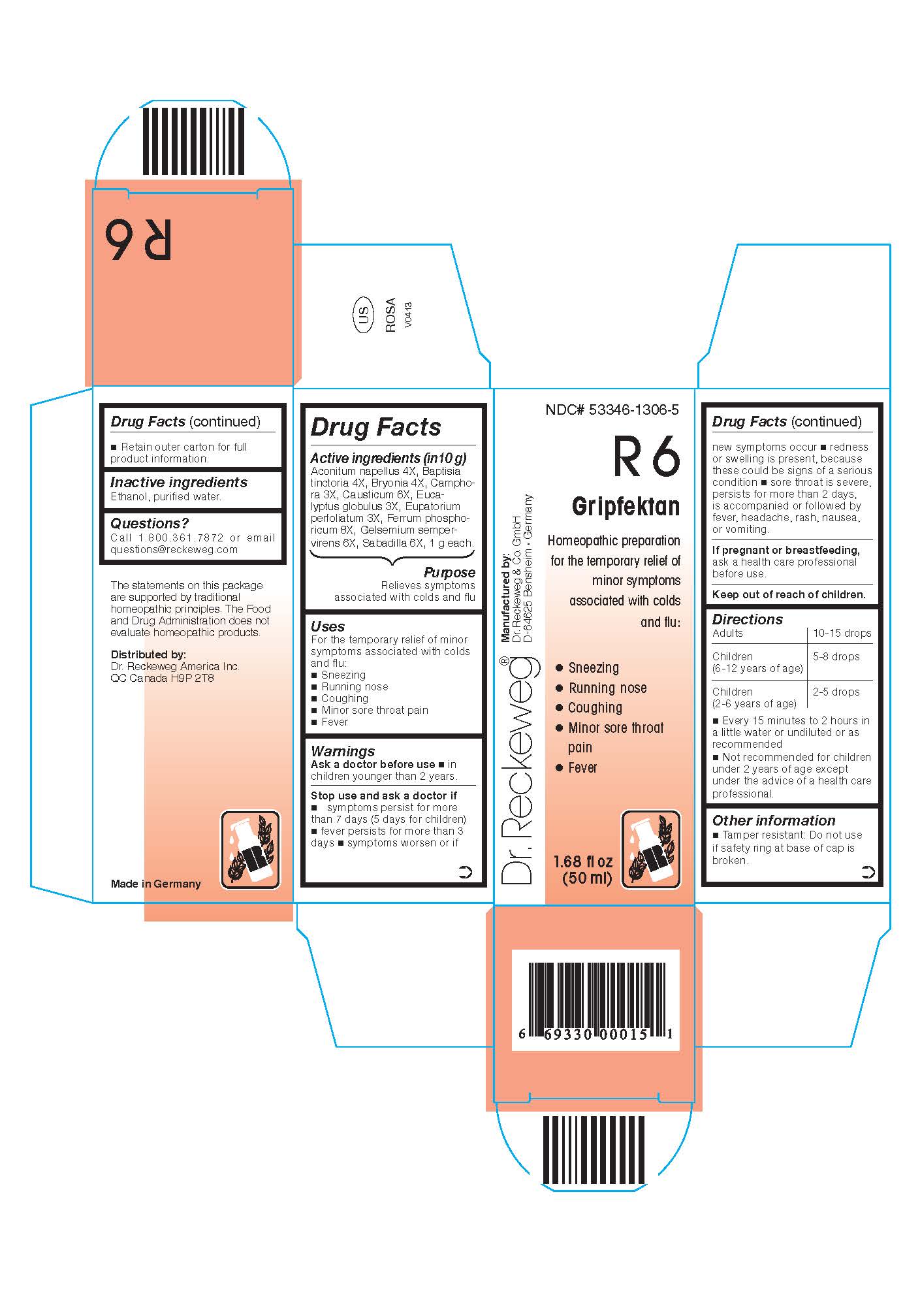
Warnings
Ask a doctor before use
* in children younger than 2 years
Stop use and ask a doctor if
* symptoms persist for more than 7 days (5 days for children)
* fever persists for more than 3 days
* symptoms worsen or if new symptoms occur
* redness or swelling is present, because these could be signs of a serious condition
* sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Uses
For the temporary relief of minor symptoms associated with colds and flu:
* Sneezing
* Running nose
* Coughing
* Minor sore throat pain
* Fever
History
There is currently no drug history available for this drug.
Other Information
There are no additional details available for this product.
Sources
Combination Product Manufacturers
-
Pharmazeutische Fabrik Dr. Reckeweg & Co
![Combination Product (Aconitum Napellus 4x, Baptisia Tinctoria 4x, Bryonia 4x, Camphora 3x, Causticum 6x, Eucalyptus Globulus 3x, Eupatorium Perfoliatum 3x, Ferrum Phosphoricum 8x, Gelsemium Sempervirens 6x, Sabadilla 6x) Liquid [Pharmazeutische Fabrik Dr. Reckeweg & Co]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Combination Product | Pharmazeutische Fabrik Dr. Reckeweg & Co
![Combination Product (Aconitum Napellus 4x, Baptisia Tinctoria 4x, Bryonia 4x, Camphora 3x, Causticum 6x, Eucalyptus Globulus 3x, Eupatorium Perfoliatum 3x, Ferrum Phosphoricum 8x, Gelsemium Sempervirens 6x, Sabadilla 6x) Liquid [Pharmazeutische Fabrik Dr. Reckeweg & Co] Combination Product (Aconitum Napellus 4x, Baptisia Tinctoria 4x, Bryonia 4x, Camphora 3x, Causticum 6x, Eucalyptus Globulus 3x, Eupatorium Perfoliatum 3x, Ferrum Phosphoricum 8x, Gelsemium Sempervirens 6x, Sabadilla 6x) Liquid [Pharmazeutische Fabrik Dr. Reckeweg & Co]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Directions
Adults: 10-15 drops; children (6-12 years of age): 5-8 drops;
children (2-6 years of age): 2-5 drops, every 15 minutes to 2 hours in a little water or undiluted or as recommended.
Not recommended for children under 2 years of age except under the advice of a health care professional.
Login To Your Free Account