FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Cosentyx Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
COSENTYXTM is indicated for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy.
History
There is currently no drug history available for this drug.
Other Information
Secukinumab is a recombinant human monoclonal IgG1/κ antibody that binds specifically to IL-17A. It is expressed in a recombinant Chinese Hamster Ovary (CHO) cell line. Secukinumab has a molecular mass of approximately 151 kDa; both heavy chains of secukinumab contain oligosaccharide chains.
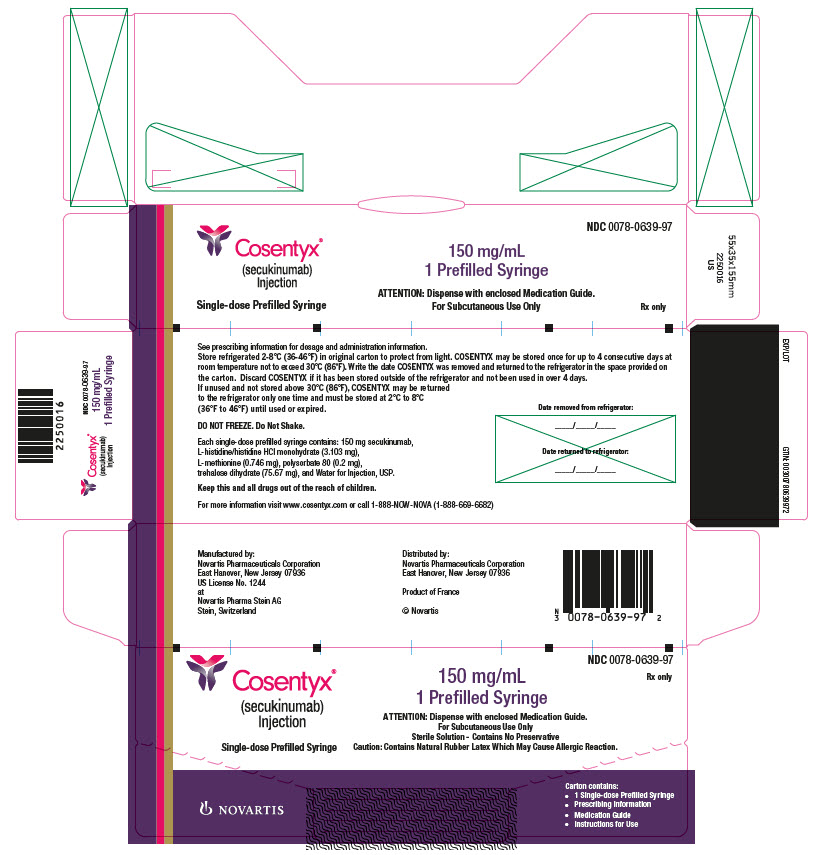
COSENTYX Injection
COSENTYX injection is a sterile, preservative-free, clear to slightly opalescent, colorless to slightly yellow solution. COSENTYX is supplied in a single-use Sensoready pen with a 27 gauge fixed ½ inch needle, or a single-use prefilled syringe with a 27 gauge fixed ½ inch needle. The removable cap of the COSENTYX Sensoready pen or prefilled syringe contains natural rubber latex.
Each COSENTYX Sensoready pen or prefilled syringe contains 150 mg of secukinumab formulated in: L-histidine/histidine hydrochloride monohydrate (3.103 mg), L-methionine (0.746 mg), polysorbate 80 (0.2 mg), trehalose dihydrate (75.67 mg), and Sterile Water for Injection, USP, at pH of 5.8.
COSENTYX for Injection
COSENTYX for injection is supplied as a sterile, preservative free, white to slightly yellow, lyophilized powder in single-use vials. Each COSENTYX vial contains 150 mg of secukinumab formulated in L-histidine/L-histidine hydrochloride monohydrate (4.656 mg), polysorbate 80 (0.6 mg), and sucrose (92.43 mg). Following reconstitution with 1 mL Sterile Water for Injection, USP, the resulting pH is approximately 5.8.
Sources
Cosentyx Manufacturers
-
Novartis Pharmaceuticals Corporation
![Cosentyx (Secukinumab) Injection [Novartis Pharmaceuticals Corporation]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Cosentyx | Novartis Pharmaceuticals Corporation
![Cosentyx (Secukinumab) Injection [Novartis Pharmaceuticals Corporation] Cosentyx (Secukinumab) Injection [Novartis Pharmaceuticals Corporation]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
2.1 Recommended DosageThe recommended dose is 300 mg by subcutaneous injection at Weeks 0, 1, 2, 3, and 4 followed by 300 mg every 4 weeks. Each 300 mg dose is given as 2 subcutaneous injections of 150 mg.
For some patients, a dose of 150 mg may be acceptable.
2.2 Important Administration InstructionsThere are three presentations for COSENTYX (i.e., Sensoready pen, prefilled syringe, and lyophilized powder in vial for reconstitution). The COSENTYX “Instructions for Use” for each presentation contains more detailed instructions on the preparation and administration of COSENTYX [see Instructions for Use].
COSENTYX is intended for use under the guidance and supervision of a physician. Patients may self-inject after proper training in subcutaneous injection technique using the Sensoready pen or prefilled syringe and when deemed appropriate. The lyophilized powder for reconstitution is for healthcare provider use only. Administer each injection at a different anatomic location (such as upper arms, thighs or any quadrant of abdomen) than the previous injection, and not into areas where the skin is tender, bruised, erythematous, indurated or affected by psoriasis. Administration of COSENTYX in the upper, outer arm may be performed by a caregiver or healthcare provider.
2.3 Preparation for Use of COSENTYX Sensoready® Pen and Prefilled SyringeBefore injection, remove COSENTYX Sensoready pen or COSENTYX prefilled syringe from the refrigerator and allow COSENTYX to reach room temperature (15 to 30 minutes) without removing the needle cap.
The removable cap of the COSENTYX Sensoready pen and the COSENTYX prefilled syringe contains natural rubber latex and should not be handled by latex-sensitive individuals [see Warnings and Precautions (5.5)].
Inspect COSENTYX visually for particulate matter and discoloration prior to administration. COSENTYX injection is a clear to slightly opalescent, colorless to slightly yellow solution. Do not use if the liquid contains visible particles, is discolored or cloudy. COSENTYX does not contain preservatives; therefore, administer the Sensoready pen or prefilled syringe within 1 hour after removal from the refrigerator. Discard any unused product remaining in the Sensoready pen or prefilled syringe.
2.4 Reconstitution and Preparation of COSENTYX Lyophilized PowderCOSENTYX lyophilized powder should be prepared and reconstituted with Sterile Water for Injection by a trained healthcare provider using aseptic technique and without interruption. The preparation time from piercing the stopper until end of reconstitution on average takes 20 minutes and should not exceed 90 minutes.
a) Remove the vial of COSENTYX lyophilized powder from the refrigerator and allow to stand for 15 to 30 minutes to reach room temperature. Ensure the Sterile Water for Injection is at room temperature.
b) Slowly inject 1 mL of Sterile Water for Injection into the vial containing COSENTYX lyophilized powder and direct the stream of Sterile Water for Injection onto the lyophilized powder.
c) Tilt the vial at an angle of approximately 45 degrees and gently rotate between the fingertips for approximately 1 minute. Do not shake or invert the vial.
d) Allow the vial to stand for about 10 minutes at room temperature to allow for dissolution. Note that foaming may occur.
e) Tilt the vial at an angle of approximately 45 degrees and gently rotate between the fingertips for approximately 1 minute. Do not shake or invert the vial.
f) Allow the vial to stand undisturbed at room temperature for approximately 5 minutes. The reconstituted COSENTYX solution should be essentially free of visible particles, clear to opalescent, and colorless to slightly yellow. Do not use if the lyophilized powder has not fully dissolved or if the liquid contains visible particles, is cloudy or discolored.
g) Prepare the required number of vials (1 vial for the 150 mg dose or 2 vials for the 300 mg dose).
h) The COSENTYX reconstituted solution contains 150 mg of secukinumab in 1 mL of solution. After reconstitution, use the solution immediately or store in the refrigerator at 2ºC to 8ºC (36ºF to 46ºF) for up to 24 hours. Do not freeze.
i) If stored at 2ºC to 8ºC (36ºF to 46ºF), allow the reconstituted COSENTYX solution to reach room temperature (15 to 30 minutes) before administration. COSENTYX does not contain preservatives; therefore, administer within 1 hour after removal from 2ºC to 8ºC (36ºF to 46ºF) storage.
Login To Your Free Account