Cyclobenzaprine Hydrochloride Manufacturers
-
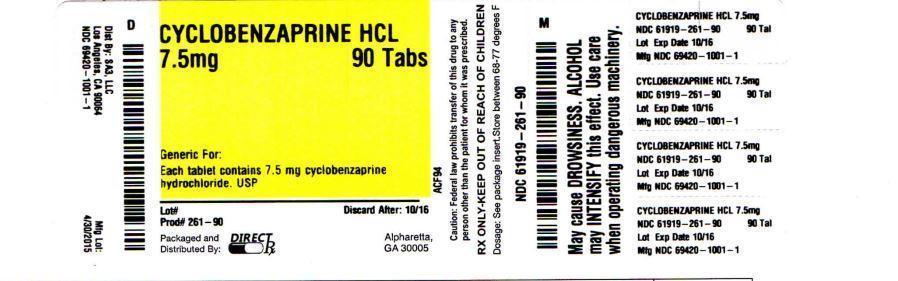
Direct Rx
-
Preferred Pharmaceuticals, Inc.
-
Preferred Pharmaceuticals, Inc.
-
Northwind Pharmaceuticals, Llc
-
Blenheim Pharmacal, Inc.
-
Remedyrepack Inc.
-
Direct Rx
-
Proficient Rx Lp
-
Direct Rx
-
Remedyrepack Inc.
-
Remedyrepack Inc.
-
Northwind Pharmaceuticals, Llc
-
Remedyrepack Inc.
-
Remedyrepack Inc.
-
Rxchange Co.
-
Remedyrepack Inc.
-
Remedyrepack Inc.
-
Rebel Distributors
-
Rebel Distributors
-
Blenheim Pharmacal, Inc.
-
Kaiser Foundation Hospitals
-
Contract Pharmacy Services-pa
-
Physicians Total Care, Inc.
-
State Of Florida Doh Central Pharmacy
-
Mckesson Packaging Services Business Unit Of Mckesson Corporation
-
Keltman Pharmaceuticals Inc.
-
Amneal Pharmaceuticals
-
Remedyrepack Inc.
-
Stat Rx Usa Llc
-
Redpharm Drug Inc.
-
Redpharm Drug Inc.
-
Bryant Ranch Prepack
-
Redpharm Drug Inc.
-
Pd-rx Pharmaceuticals, Inc.
-
Pd-rx Pharmaceuticals, Inc.
-
Stat Rx Usa Llc
-
Pd-rx Pharmaceuticals, Inc.
-
Stat Rx Usa Llc
-
Lake Erie Medical & Surgical Supply Dba Quality Care Products Llc
-
Remedyrepack Inc.
-
Breckenridge Pharmaceutical, Inc.
-
Lake Erie Medical & Surgical Supply Dba Quality Care Products Llc
-
Camber Pharmaceuticals
-
Mckesson Contract Packaging
-
Major Pharmaceuticals
-
Bryant Ranch Prepack
-
Unit Dose Services
-
Remedyrepack Inc.
-
Remedyrepack Inc.
-
Remedyrepack Inc.
-
Jubilant Cadista Pharmaceuticals, Inc.
-
Qualitest Pharmaceuticals
-
Kle 2, Inc.
-
Mutual Pharmaceutical
-
Watson Laboratories, Inc.
-
Remedyrepack Inc.
-
Remedyrepack Inc.
-
Remedyrepack Inc.
-
Remedyrepack Inc.
-
Remedyrepack Inc.
-
Golden State Medical Supply, Inc.
-
Clinical Solutions Wholesale
-
Dispensing Solutions, Inc.
-
Pd-rx Pharmaceuticals, Inc.
-
Pliva Inc.
-
Dispensing Solutions, Inc.
-
Ranbaxy Pharmaceuticals Inc
-
Lake Erie Medical Dba Quality Care Product Llc
-
Pharmaceutica North America, Inc.
-
Dispensing Solutions, Inc.
-
Lake Erie Medical Dba Quality Care Products Llc
-
Medvantx, Inc.
-
Liberty Pharmaceuticals, Inc.
-
Clinical Solutions Wholesale
-
Cima Labs Inc
-
Ncs Healthcare Of Ky, Inc Dba Vangard Labs
-
Unit Dose Services
-
Remedyrepack Inc.
-
Aurobindo Pharma Limited
-
New Horizon Rx Group, Llc
-
Aidarex Pharmaceuticals Llc
-
Aidarex Pharmaceuticals Llc
-
American Health Packaging
-
Mckesson Packaging Services A Business Unit Of Mckesson Corporation
-
Remedyrepack Inc.
-
Direct Rx
-
Blenheim Pharmacal, Inc.
-
Mylan Pharmaceuticals Inc.
-
Pd-rx Pharmaceuticals, Inc.
-
State Of Florida Doh Central Pharmacy
-
State Of Florida Doh Central Pharmacy
-
Mylan Institutional Inc.
-
Proficient Rx Lp
-
Legacy Pharmaceutical Packaging
-
Readymeds
-
Northwind Pharmaceuticals
-
Lake Erie Medical Dba Quality Care Products Llc
-
American Health Packaging
-
Proficient Rx Lp
-
Proficient Rx Lp
-
Aphena Pharma Solutions – Tennessee, Llc
-
Remedyrepack Inc.
-
Kvk-tech, Inc.
-
Pd-rx Pharmaceuticals, Inc.
-
Preferred Pharmaceuticals, Inc.
-
Preferred Pharmaceuticals, Inc.
-
Avpak
-
Preferred Pharmaceuticals, Inc.
-
Cardinal Health
-
Proficient Rx Lp
-
State Of Florida Doh Central Pharmacy
-
Medsource Pharmaceuticals
-
Solubiomix
-
Medsource Pharmaceuticals
-
Remedyrepack Inc.
-
Remedyrepack Inc.
-
Remedyrepack Inc.
Login To Your Free Account


![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Preferred Pharmaceuticals, Inc.]](https://www.recallguide.org/wp-content/themes/bootstrap/assets/img/drug-image-placeholder.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Preferred Pharmaceuticals, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=9bc56d83-e1a7-49c3-a869-4d16c24a5257&name=8b931a82-figure-03.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Northwind Pharmaceuticals, Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=0038f081-a867-43ed-8415-9f0003871291&name=51655-442-52.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Blenheim Pharmacal, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=119094a0-1c9a-49cf-e054-00144ff88e88&name=LABEL_CYCLOBENZAPRINEHCLTABS5MG_BPI(10544-200-30)_KVK(10702-006-01)_REV2.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=a3fdbbf0-574b-4ccc-9389-ea1d12a49bc5&name=MM2.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Direct Rx]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=6415dae5-c716-4901-9639-d17960229dea&name=extra-stregnth-gas-relief-1.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Proficient Rx Lp]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=d2361d16-94fc-43a9-8009-ed03dce0544f&name=d2361d16-94fc-43a9-8009-ed03dce0544f-02.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Direct Rx]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=650a8b39-28fa-4515-a2fb-b2989e2331da&name=label.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=e2cacd44-dcf9-43de-b308-c0af0dc26604&name=MM2.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=bec430f9-63a6-4752-9d2b-87415bc1cf37&name=MM2.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Northwind Pharmaceuticals, Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=239ba279-9ac2-4edc-8e0b-697e16951986&name=hyd0g-0000-01.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=206c072d-a6a9-49eb-9741-5d08353d557b&name=MM3.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=a264aaca-4162-46f7-bac8-904b55322560&name=MM2.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Rxchange Co.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=7b9f9a11-74e6-4198-965d-78ea36c9cec9&name=CycloBenzaprineHCl.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=37ed3a36-36e7-4eb1-803e-0f551273e572&name=MM2.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=e94df5c4-d6da-4a89-958e-e84deb029ad0&name=sleepnowremedyjpg.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Rebel Distributors]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=0e185b89-d7cc-477f-ab8c-7c6f44e5c9e7&name=037.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Rebel Distributors]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=dd2d3cf8-293c-4c3b-bdce-81f5c6a220ef&name=036.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Blenheim Pharmacal, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=f4fee702-2561-4317-b219-23d4543f2c75&name=10544-159-20-65162-541-11.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Kaiser Foundation Hospitals]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=c21a7f59-09c3-485a-b6d9-3b784b7e9042&name=cyclobenzaprine-02.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Contract Pharmacy Services-pa]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=e9063c42-e71a-4cce-9cd9-9ed6a192c6bb&name=e9063c42-e71a-4cce-9cd9-9ed6a192c6bb-01.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Physicians Total Care, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=4eddf137-fd00-43d0-96ed-16f057806d4e&name=10mgpackagelabel.jpg)
![Cyclobenzaprine Hydrochloride Tablet [State Of Florida Doh Central Pharmacy]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=54a6b01c-5a7c-4710-93fa-69fd2c2549e3&name=Cyclobenzaprine10mg(Amneal).jpg)
![Cyclobenzaprine Hydrochloride Tablet [Mckesson Packaging Services Business Unit Of Mckesson Corporation]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=61b5704e-b635-4e3f-b6f9-a7faf5d3b6b6&name=61b5704e-b635-4e3f-b6f9-a7faf5d3b6b6-01.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Keltman Pharmaceuticals Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=220ebc47-d310-4668-8b51-47a4981b46ef&name=cyclobenzaprine_10mg.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Amneal Pharmaceuticals]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=916339d0-aee1-425c-a5f1-8d0217699220&name=gentle-laxative-1.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=baa6d111-4222-4fa8-b7d2-295310177d96&name=MM2.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Stat Rx Usa Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=c68204a7-d00c-4ce7-bfda-bff58b2ed67e&name=CYCLOBENZAPRINE5MGLABEL544.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Redpharm Drug Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=ee3f98d0-71bb-42d3-b863-5bf92adf6353&name=6729601061.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Redpharm Drug Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=1534e79c-12cf-493e-8034-c1083f900ff5&name=6729600532.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Bryant Ranch Prepack]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=bd89c175-7eb7-6abf-d30f-c6563ec0a525&name=label1datamaxfda375.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Redpharm Drug Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=fae7e4a7-ca63-4af4-a43a-c5d85cce1961&name=6729604474.jpg)
![Cyclobenzaprine Hydrochloride (Cyclobenzaprine Hydrochloride) Tablet [Pd-rx Pharmaceuticals, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=6b3eccd8-2078-47d6-ac69-e7206ca27914&name=55289567.jpg)
![Cyclobenzaprine Hydrochloride (Cyclobenzaprine Hydrochloride) Tablet [Pd-rx Pharmaceuticals, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=bb7bf4f3-6806-4f37-b61b-13407f759b36&name=43063050.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Stat Rx Usa Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=a4187ac9-cdef-4a65-8966-e329acbe9ee2&name=8af4c089-figure-01.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Pd-rx Pharmaceuticals, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=94dbab72-615e-45c9-bb05-70748b4b6aaa&name=55289236.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Stat Rx Usa Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=59500ba3-b16e-46f9-a867-6c54f1f8fd6a&name=Cyclobenzaprine10mg508.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Lake Erie Medical & Surgical Supply Dba Quality Care Products Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=8b9f1984-cce8-4b5b-baee-77ba9eff2a56&name=cyclobenzaprinehcl5mgwatson.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=206ea94a-8386-4d65-a74e-8abd52e1bfc3&name=MM2.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated Cyclobenzaprine Hydrochloride Tablet, Coated [Breckenridge Pharmaceutical, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=97c78d60-3498-476f-924a-b76078c5f97a&name=cyclobenzaprine-02.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Lake Erie Medical & Surgical Supply Dba Quality Care Products Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=3f0512a2-66ef-453d-b0c5-ac58c3cd5c95&name=CyclobenzaprineHCl10mgWatson.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Camber Pharmaceuticals]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=68347db5-9f3d-430e-bd8a-16071077648f&name=8b931a82-figure-01.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Mckesson Contract Packaging]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=19567e4e-2afe-41e7-b8d1-956843b75687&name=19567e4e-2afe-41e7-b8d1-956843b75687-02.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Major Pharmaceuticals]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=092e2b22-37b3-4bea-9945-6480121175ce&name=cyclobenzaprine-hci-2.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Bryant Ranch Prepack]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=49f500af-0ee8-4384-91d9-8822cffac976&name=label1datamaxfda188.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Unit Dose Services]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=639dc006-495f-4160-9258-babf4ef3c5d3&name=uds4842.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=6ea47f30-1fbd-4342-bb43-72238558769b&name=MM2.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=7934ef51-ba40-4779-a993-64ad7a85e818&name=MM2.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=d9f02f87-cee2-41c9-a03a-c745ae5a380a&name=MM2.jpg)
![Cyclobenzaprine Hydrochloride (Cyclobenzaprine Hydrochloride) Tablet [Jubilant Cadista Pharmaceuticals, Inc.]](http://recallguide.cwdevelopsp.com/wp-content/themes/recallguide/assets/img/drug-image-placeholder.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Qualitest Pharmaceuticals]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=6ecd47d4-c618-48b1-a38d-1d558d54fd09&name=cyclobenzaprine-2.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Mutual Pharmaceutical]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=ac490da0-6347-4f5d-bcae-c23261b43725&name=cyclobenzaprine-01a.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Watson Laboratories, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=aa5af84e-428e-47ee-9f4e-41dac29d1979&name=cyclobenzaprine-hydrochloride-tablets-usp-2.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=8e7fcbed-2776-4797-9850-f854da7170de&name=MM2.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=e58cfbd7-77ba-425b-88c5-d783ce66ea18&name=MM2.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=fda50d4a-6e4c-40eb-a55d-263f12191f22&name=MM2.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=e2e28f29-62a1-4bc4-a8bc-90b5614fa79b&name=MM2.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=6b4e61d9-88ce-4a7a-b2ab-d0ff8b15fd72&name=MM2.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Golden State Medical Supply, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=eeff0c85-985f-4333-80f0-66e8fc31d37f&name=LabelGraphic-10mg100s.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Clinical Solutions Wholesale]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=01b14c0c-b302-4ab3-bffa-0aa0eae17ee4&name=Cycl5Lab08.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Dispensing Solutions, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=bb207c2e-87b3-4d5d-896a-e7e4afe33124&name=NDC66336-0581-XX-----Watson.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Pd-rx Pharmaceuticals, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=6a8b14a6-84ec-47ed-9555-04605960a71e&name=43063460.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Pliva Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=a88fa950-23d9-49fd-9340-b38a357760bf&name=8c5b9804-2e8b-4e96-95b3-75d13d6ca1e2-02.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Dispensing Solutions, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=fa09ea71-504b-49ff-b269-f124e8eeff26&name=NDC68258-7150-XX------KLE.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Ranbaxy Pharmaceuticals Inc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=fa8ea64a-62e7-4bbc-a4d3-4fee53cf21c7&name=label.jpg)
![Cyclobenzaprine Hydrochloride (Cyclobenzaprine Hydrochloride) Tablet, Film Coated [Lake Erie Medical Dba Quality Care Product Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=c738fb6a-bdde-4149-97e4-de8e63236e7a&name=cyclobenzaprinehcl10mgamneal.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Pharmaceutica North America, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=f7dae878-f0bd-4578-9ed1-d2f16f5823c2&name=CyclobenzaprineLabel.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Dispensing Solutions, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=7fffc1a1-fd8e-450f-a48f-f91ab3c656ca&name=NDC68258-7013-XX------QUALITEST.jpg)
![Cyclobenzaprine Hydrochloride Tablet Cyclobenzaprine Hydrochloride (Cyclobenzaprine Hydrochloride) Tablet [Lake Erie Medical Dba Quality Care Products Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=9ec8690d-5983-4e20-ab5d-700f5a7bc1cc&name=CyclobenzaprineHCL5mgJubilantCadista.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Medvantx, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=6a682677-4a99-43a5-a991-af7f2d69aca1&name=6a682677-4a99-43a5-a991-af7f2d69aca1-02.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Liberty Pharmaceuticals, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=4636827c-a0cf-46e3-a682-08a19021be11&name=cyclobenzaprine-for-watson-2.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Clinical Solutions Wholesale]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=9e160812-83dc-4b8b-9d52-dc94c513538d&name=e496ff89-97f6-4088-8ade-02159b4d97bb-02.jpg)
![Cyclobenzaprine Hydrochloride Capsule, Extended Release [Cima Labs Inc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=6d549942-9f30-4b4c-b6ae-c7d63fec779f&name=3e10f595-f026-4bfc-a0db-d6d135c80f78-02.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Ncs Healthcare Of Ky, Inc Dba Vangard Labs]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=d787900f-f617-478d-a65b-eef170e929f9&name=image-02.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Unit Dose Services]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=3dfb1c4d-3ffe-4ffb-918e-1f7d07f5011e&name=48aae6a0-4d16-46ef-95d4-e301ee26ccbc.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=6c6a3831-4712-4c23-b00f-7fc63085abe6&name=MM2.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Aurobindo Pharma Limited]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=1154688c-1383-442b-a3a5-ec01e038795c&name=cyclobenz-fig1.jpg)
![Cyclobenzaprine Hydrochloride Tablet [New Horizon Rx Group, Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=dcd0bfcd-9625-4712-9c43-37b533aea7bb&name=e8799c4d-figure-01.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Aidarex Pharmaceuticals Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=61d0567c-03d6-4fe6-a239-97c3e137559f&name=cyclobenzaprine-hcl-7-5mg-tab-for-kle-2.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Aidarex Pharmaceuticals Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=c108987e-f1f9-4f0e-893a-fb036af0ce08&name=cyclobenzaprine-hcl-10mg-for-amneal-2.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [American Health Packaging]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=47bed841-47a2-4112-9b80-7b978c90593e&name=14cc23fe-9ea0-4118-ab76-cfa1e9705401-02.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Mckesson Packaging Services A Business Unit Of Mckesson Corporation]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=e1faa896-cd20-4cc5-aa95-1797dc590917&name=ac09241f-3425-4c26-8401-73b093ca13a6-02.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=f0830c1a-ee35-4285-b6db-fdbd6fbdb82a&name=MM2.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Blenheim Pharmacal, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=7c6e9536-54a3-431d-b92d-39e8e6a0902b&name=MontelukastSodiumTablets10mg30TabletsRev0714.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Mylan Pharmaceuticals Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=b891bd9f-fdb8-4862-89c5-ecdd700398a3&name=image-07.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Pd-rx Pharmaceuticals, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=19a60d50-7319-44d9-9ade-72922cfcb39d&name=image-02.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [State Of Florida Doh Central Pharmacy]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=c62f6022-aafd-456e-b52c-99cd1cc5c3c6&name=Cyclobenzaprine10mg.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [State Of Florida Doh Central Pharmacy]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=ab3f3674-e0ee-473c-9e07-4a836f0603b6&name=Cyclobenzaprine10mg.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Mylan Institutional Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=9875e2a2-e8d6-430f-8eb6-c0103ad276db&name=15e06932-06cc-4c53-81ed-a0457f1168f7-02.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Proficient Rx Lp]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=46100534-edd7-408e-879a-bab1c8fd9e3e&name=cyclobenzaprine-hydrochloride-tablets-usp-2.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Legacy Pharmaceutical Packaging]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=a56a571b-d556-45d1-8a2a-a51d459183dc&name=cyclobenzaprine-hcl-tablets-2.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Readymeds]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=ad306e55-6c89-4d14-a553-2ac776b4dd28&name=8af4c089-figure-01.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Northwind Pharmaceuticals]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=0e2c0580-49d7-4bd5-85df-7a3591198ab0&name=Cyclobenzaprine51655-439.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Lake Erie Medical Dba Quality Care Products Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=3e335564-5f25-494e-a90e-e4c38460b9fd&name=CyclobenzaprineHCL5mgQualitest.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [American Health Packaging]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=5f321a03-ccfb-4c11-ac60-a461a5580c69&name=CyclobenzaprineHydrochloridetablets,USP5mglabel.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Proficient Rx Lp]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=f14bc532-e30f-490f-b97b-d0dab9a2ba0c&name=cyclobenzaprine-03.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Proficient Rx Lp]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=d88c45a4-7478-497d-93e1-15629181f650&name=Cyclobenzaprine.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Aphena Pharma Solutions – Tennessee, Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=1d325ea3-fd3f-4cf7-9d07-d5144e7945f5&name=67544-251.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=0e2b7211-de3a-4468-bf86-03075ad0befd&name=MM2.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Kvk-tech, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=726ce482-e6c2-49dc-b46c-284969c23835&name=cvs-44-531a-1.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Preferred Pharmaceuticals, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=7ccfab67-8393-4af0-9023-97a785f7cb6f&name=d448f424-1370-4077-8f33-f78659b2b2c9-02.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Preferred Pharmaceuticals, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=e2f44c02-d3aa-4f12-bedb-2497cdc28a58&name=163c8ef5-cf00-4045-a2f8-36fd1906eea0-02.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Avpak]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=64f6d1be-4067-a5c7-2343-4aed651a0cac&name=8b931a82-figure-01.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Preferred Pharmaceuticals, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=50c524ab-7029-494a-8bb4-d90a4d184fb2&name=box1.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Cardinal Health]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=6b470377-a5b9-494f-abaf-c0454b5108b9&name=image-01.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Proficient Rx Lp]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=50aa421f-1d73-4b43-8528-2617ef2507dc&name=8af4c089-figure-02.jpg)
![Cyclobenzaprine Hydrochloride Tablet [State Of Florida Doh Central Pharmacy]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=879a4e55-012d-4988-b571-9a8cf94414dd&name=10mgcadista.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Medsource Pharmaceuticals]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=1db200b7-75d2-5e17-e054-00144ff8d46c&name=433-60.jpg)
![Cyclobenzaprine Hydrochloride (Cyclobenzaprine Hydrochloride Tablet, Film Coated) Tablet [Solubiomix]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=1d539e9b-c30e-44f4-a579-7ee10976ee12&name=tussin-dm-cough-chest-congestion-1.jpg)
![Cyclobenzaprine Hydrochloride Tablet, Film Coated [Medsource Pharmaceuticals]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=1f915bbb-93a1-6cfd-e054-00144ff8d46c&name=685-60.jpg)
![Cyclobenzaprine Hydrochloride Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=0bb20391-f750-414a-98b5-4636c6809be5&name=MM2.jpg)