FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Dayrelief Pe Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Calcium Acetate Capsule is a phosphate binder indicated to reduce serum phosphorus in patients with end stage renal disease (ESRD).
History
There is currently no drug history available for this drug.
Other Information
Calcium Acetate Capsule acts as a phosphate binder. Its chemical name is calcium acetate. Its molecular formula is C4H6CaO4, and its molecular weight is 158.17. Its structural formula is:
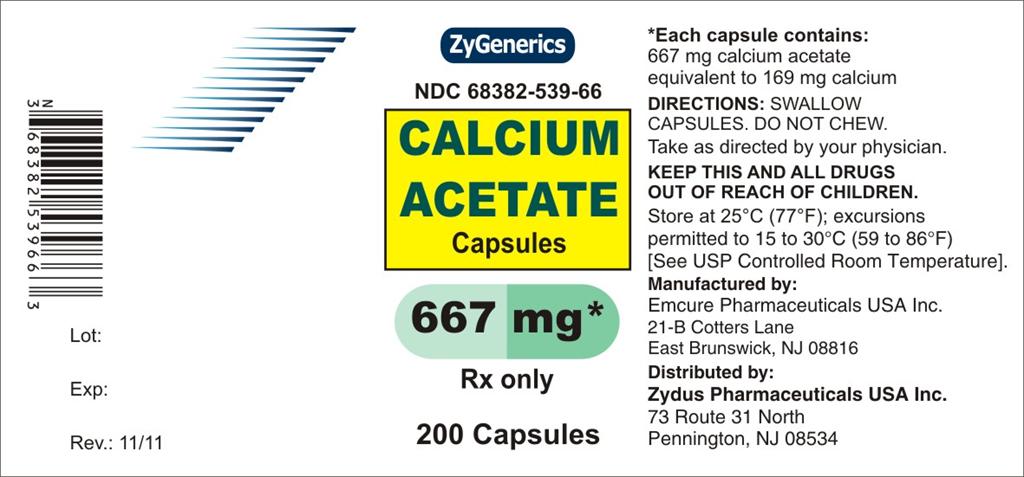
Calcium Acetate Capsules are Hard Gelatin Capsules with blue opaque cap and body both imprinted "EP 115" in black ink. Each capsule contains 667 mg calcium acetate, USP (anhydrous; Ca(CH3COO)2; MW=158.17 grams) equal to 169 mg (8.45 mEq) calcium and following inactive ingredients crospovidone, FD & C Blue No. 1, FD & C Blue No. 3, gelatin, magnesium stearate, sodium lauryl sulfate and titanium dioxide. In addition to the ingredients listed above, each capsule contains following inactive ingredients from imprinting ink: butyl alcohol, iron oxide black, propylene glycol and shellac.
Sources
Dayrelief Pe Manufacturers
-
Western Family Foods Inc
![Dayrelief Pe (Acetaminophen, Dextromethorphan Hydrobromide, Phenylephrine Hydrochloride) Capsule, Liquid Filled [Western Family Foods Inc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Dayrelief Pe | Zydus Pharmaceuticals Usa Inc.
![Dayrelief Pe (Acetaminophen, Dextromethorphan Hydrobromide, Phenylephrine Hydrochloride) Capsule, Liquid Filled [Western Family Foods Inc] Dayrelief Pe (Acetaminophen, Dextromethorphan Hydrobromide, Phenylephrine Hydrochloride) Capsule, Liquid Filled [Western Family Foods Inc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
The recommended initial dose of Calcium Acetate Capsules for the adult dialysis patient is 2 capsules with each meal. Increase the dose gradually to lower serum phosphorus levels to the target range, as long as hypercalcemia does not develop. Most patients require 3-4 capsules with each meal.
Login To Your Free Account