Elidel Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
WARNING
Long-term Safety of Topical Calcineurin Inhibitors Has Not Been Established
Although a causal relationship has not been established, rare cases of malignancy (e.g., skin and lymphoma) have been reported in patients treated with topical calcineurin inhibitors, including ELIDEL Cream.
Therefore:
- Continuous long-term use of topical calcineurin inhibitors, including ELIDEL Cream, in any age group should be avoided, and application limited to areas of involvement with atopic dermatitis.
- ELIDEL Cream is not indicated for use in children less than 2 years of age.
Prolonged systemic use of calcineurin inhibitors for sustained immunosuppression in animal studies and transplant patients following systemic administration has been associated with an increased risk of infections, lymphomas, and skin malignancies. These risks are associated with the intensity and duration of immunosuppression.
Based on this information and the mechanism of action, there is a concern about a potential risk with the use of topical calcineurin inhibitors, including ELIDEL Cream. While a causal relationship has not been established, rare cases of skin malignancy and lymphoma have been reported in patients treated with topical calcineurin inhibitors, including ELIDEL Cream. Therefore:
- ELIDEL Cream should not be used in immunocompromised adults and children.
- If signs and symptoms of atopic dermatitis do not improve within 6 weeks, patients should be re-examined by their healthcare provider and their diagnosis be confirmed (see PRECAUTIONS).
- The safety of ELIDEL Cream has not been established beyond one year of non-continuous use.
(See CLINICAL PHARMACOLOGY, WARNINGS, boxed WARNING, PRECAUTIONS, INDICATIONS AND USAGE, and DOSAGE AND ADMINISTRATION.)
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
ELIDEL ® (pimecrolimus) Cream 1% is indicated as second-line therapy for the short-term and non-continuous chronic treatment of mild to moderate atopic dermatitis in non-immunocompromised adults and children 2 years of age and older, who have failed to respond adequately to other topical prescription treatments, or when those treatments are not advisable.
ELIDEL Cream is not indicated for use in children less than 2 years of age (see WARNINGS, boxed WARNING, and PRECAUTIONS, Pediatric Use).
History
There is currently no drug history available for this drug.
Other Information
ELIDEL ® (pimecrolimus) Cream 1% contains the compound pimecrolimus, the immunosuppressant 33-epi-chloro-derivative of the macrolactam ascomycin.
Chemically, pimecrolimus is (1R,9S,12S,13R,14S,17R,18E,21S,23S,24R,25S,27R)-12-[(1E)-2-{(1R,3R,4S)-4-chloro-3-methoxycyclohexyl}-1-methylvinyl]-17-ethyl-1,14-dihydroxy-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-aza-tricyclo[22.3.1.0 4,9]octacos-18-ene-2,3,10,16-tetraone.
The compound has the empirical formula C 43H68CINO11 and the molecular weight of 810.47. The structural formula is
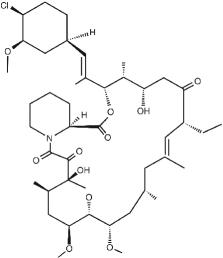
Pimecrolimus is a white to off-white fine crystalline powder. It is soluble in methanol and ethanol and insoluble in water.
Each gram of ELIDEL Cream 1% contains 10 mg of pimecrolimus in a whitish cream base of benzyl alcohol, cetyl alcohol, citric acid, mono- and di-glycerides, oleyl alcohol, propylene glycol, sodium cetostearyl sulphate, sodium hydroxide, stearyl alcohol, triglycerides, and water.
Sources