Emtriva Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
EMTRIVA® is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection.
Additional important information regarding the use of EMTRIVA for the treatment of HIV-1 Infection:
- EMTRIVA should not be coadministered with ATRIPLA®, TRUVADA®, or lamivudine-containing products [See Warnings and Precautions (5.3)].
- In treatment-experienced patients, the use of EMTRIVA should be guided by laboratory testing and treatment history [See Clinical Pharmacology (12.4)].
History
There is currently no drug history available for this drug.
Other Information
EMTRIVA is the brand name of emtricitabine, a synthetic nucleoside analog with activity against human immunodeficiency virus type 1 (HIV-1) reverse transcriptase.
The chemical name of emtricitabine is 5-fluoro-1-(2R,5S)-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Emtricitabine is the (-) enantiomer of a thio analog of cytidine, which differs from other cytidine analogs in that it has a fluorine in the 5-position.
It has a molecular formula of C8H10FN3O3S and a molecular weight of 247.24. It has the following structural formula:
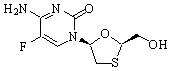
Emtricitabine is a white to off-white powder with a solubility of approximately 112 mg/mL in water at 25 °C. The log P for emtricitabine is -0.43 and the pKa is 2.65.
EMTRIVA is available as capsules or as an oral solution.
EMTRIVA capsules are for oral administration. Each capsule contains 200 mg of emtricitabine and the inactive ingredients, crospovidone, magnesium stearate, microcrystalline cellulose, and povidone.
EMTRIVA oral solution is for oral administration. One milliliter (1 mL) of EMTRIVA oral solution contains 10 mg of emtricitabine in an aqueous solution with the following inactive ingredients: cotton candy flavor, FD&C yellow No. 6, edetate disodium, methylparaben, and propylparaben (added as preservatives), sodium phosphate (monobasic), propylene glycol, water, and xylitol (added as a sweetener). Sodium hydroxide and hydrochloric acid may be used to adjust pH.
Sources




![Emtriva (Emtricitabine) Capsule [State Of Florida Doh Central Pharmacy]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=d99086c9-41a4-4c69-b41a-80054e828095&name=Emtriva200mg(Gilead).jpg)
![Emtriva (Emtricitabine) Capsule [Physicians Total Care, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=39ae3070-9b9b-41f3-b058-2e240b4ac18e&name=4853.jpg)
![Emtriva (Emtricitabine) Capsule Emtriva (Emtricitabine) Solution [Gilead Sciences, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=d6599395-3944-44f9-97f2-e0424c6b6a1f&name=emtriva-04.jpg)