Entacapone Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
Monoamine oxidase (MAO) and COMT are the two major enzyme systems involved in the metabolism of catecholamines. It is theoretically possible, therefore, that the combination of entacapone and a non-selective MAO inhibitor (e.g., phenelzine and tranylcypromine) would result in inhibition of the majority of the pathways responsible for normal catecholamine metabolism. For this reason, patients should ordinarily not be treated concomitantly with entacapone and a non-selective MAO inhibitor.
Entacapone can be taken concomitantly with a selective MAO-B inhibitor (e.g., selegiline).
Drugs Metabolized By Catechol-O-Methyltransferase (COMT)
When a single 400 mg dose of entacapone was given with intravenous isoprenaline (isoproterenol) and epinephrine without coadministered levodopa and dopa decarboxylase inhibitor, the overall mean maximal changes in heart rate during infusion were about 50% and 80% higher than with placebo, for isoprenaline and epinephrine, respectively.
Therefore, drugs known to be metabolized by COMT, such as isoproterenol, epinephrine, norepinephrine, dopamine, dobutamine, alpha-methyldopa, apomorphine, isoetherine, and bitolterol should be administered with caution in patients receiving entacapone regardless of the route of administration (including inhalation), as their interaction may result in increased heart rates, possible arrhythmias, and excessive changes in blood pressure.
Ventricular tachycardia was noted in one 32-year-old healthy male volunteer in an interaction study after epinephrine infusion and oral entacapone administration. Treatment with propranolol was required. A causal relationship to entacapone administration appears probable but cannot be attributed with certainty.
Falling Asleep During Activities of Daily Living and Somnolence
Patients with Parkinson’s disease treated with entacapone, which increases plasma levodopa levels, or with levodopa have reported suddenly falling asleep without prior warning of sleepiness while engaged in activities of daily living (including the operation of motor vehicles). Some of these episodes resulted in accidents. Although many of these patients reported somnolence while on entacapone, some did not perceive warning signs, such as excessive drowsiness, and believed that they were alert immediately prior to the event. Some of these events have been reported as late as one year after initiation of treatment.
The risk of somnolence was increased (entacapone 2% and placebo 0%) in controlled studies. It has been reported that falling asleep while engaged in activities of daily living always occurs in a setting of pre-existing somnolence, although patients may not give such a history. For this reason, prescribers should reassess patients for drowsiness or sleepiness especially since some of the events occur well after the start of treatment. Prescribers should also be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities. Patients should be advised to exercise caution while driving, operating machines, or working at heights during treatment with entacapone. Patients who have already experienced somnolence and/or an episode of sudden sleep onset should not participate in these activities during treatment with entacapone.
Before initiating treatment with entacapone, advise patients of the potential to develop drowsiness and specifically ask about factors that may increase this risk such as concomitant use of sedating medications and the presence of sleep disorders. If a patient develops daytime sleepiness or episodes of falling asleep during activities that require active participation (e.g., conversations, eating, etc.), entacapone should ordinarily be discontinued (see DOSAGE AND ADMINISTRATION for guidance on discontinuing entacapone). If the decision is made to continue entacapone, patients should be advised not to drive and to avoid other potentially dangerous activities. There is insufficient information to establish whether dose reduction will eliminate episodes of falling asleep while engaged in activities of daily living.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Entacapone tablets, USP are indicated as an adjunct to levodopa and carbidopa to treat end-of-dose “wearing-off” in patients with Parkinson’s disease (see CLINICAL PHARMACOLOGY, Clinical Studies).
Entacapone tablets, USP effectiveness has not been systematically evaluated in patients with Parkinson’s disease who do not experience end-of-dose “wearing-off”.
History
There is currently no drug history available for this drug.
Other Information
Entacapone is available as tablets containing 200 mg entacapone USP.
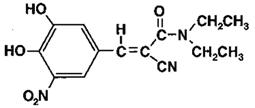
Entacapone is an inhibitor of catechol-O-methyltransferase (COMT), used in the treatment of Parkinson’s disease as an adjunct to levodopa and carbidopa therapy. It is a nitrocatechol-structured compound with a relative molecular mass of 305.29. The chemical name of entacapone is (E)-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethyl-2-propenamide. Its molecular formula is C14H15N3O5 and its structural formula is:
The inactive ingredients of the entacapone tablets, USP are croscarmellose sodium, glycerin, hydrogenated vegetable oil, hypromellose, iron oxide red, iron oxide yellow, lactose monohydrate, magnesium stearate, mannitol, microcrystalline cellulose, polysorbate, sodium starch glycolate, sucrose, and titanium dioxide.
Sources



![Entacapone Tablet, Film Coated [Kaiser Foundation Hospitals]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=af3ea4f7-7c71-4fd5-8557-9c65ce95606e&name=entacapone-02.jpg)
![Entacapone Tablet, Film Coated [Sun Pharma Global Fze]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=ad6a2b73-29d4-4ced-ba42-4c2ad1c5a1af&name=label.jpg)
![Entacapone Tablet, Film Coated [Wockhardt Limited]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=5a68fa36-ed25-4309-8624-cd6a0001ec7f&name=entacaptaborion-figure-01.jpg)
![Entacapone Tablet, Film Coated [Wockhardt Usa Llc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=3b19e8e3-336c-4aef-8721-ed121e216155&name=entacaptaborion-figure-02.jpg)
![Entacapone Tablet, Film Coated [Wockhardt Limited]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=b9ac99ad-7e82-4064-8c66-214ac6d1776b&name=entacaponetab-m-figure-02.jpg)
![Entacapone Tablet, Film Coated [Wockhardt Usa Llc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=01ea62f4-bfac-485a-bb4b-a9abb345749f&name=entacaponetab-figure-02.jpg)
![Entacapone Tablet, Film Coated [Sun Pharma Global Fze]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=205522fb-bb0e-414e-a413-a08bef727653&name=entacapone-lbl.jpg)
![Entacapone Tablet, Film Coated [Wockhardt Limited]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=f34a7de7-488c-4543-9518-2abe8e651c88&name=l1-entacaptaborion-m-figure-02.jpg)
![Entacapone Tablet, Film Coated [Wockhardt Usa Llc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=050eeb2b-a7ba-489a-9e59-a5074ff5f410&name=l1-entacaptaborion-figure-02.jpg)
![Entacapone Tablet, Film Coated [Mylan Pharmaceuticals Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=e41bbc90-bb83-4514-a38f-994e9fa76472&name=image-02.jpg)
![Entacapone Tablet, Film Coated [Mylan Institutional Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=82fb6c54-3571-4ea1-9a1b-c21418133e84&name=82fb6c54-3571-4ea1-9a1b-c21418133e84-02.jpg)
![Entacapone Tablet, Film Coated [Sandoz Inc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=ad4b6d79-efd3-4c43-b4dc-5afdacf939a4&name=ad4b6d79-efd3-4c43-b4dc-5afdacf939a4-02.jpg)
![Entacapone Tablet, Film Coated [Avkare, Inc.]](http://recallguide.cwdevelopsp.com/wp-content/themes/recallguide/assets/img/drug-image-placeholder.jpg)