Epiduo Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
EPIDUO gel is indicated for the topical treatment of acne vulgaris in patients 12 years of age and older.
History
There is currently no drug history available for this drug.
Other Information
EPIDUO (adapalene and benzoyle peroxide) gel, 0.1%/2.5% is a white to very pale yellow, opaque gel for topical use containing adapalene 0.1% and benzoyl peroxide 2.5%.
Adapalene, a synthetic retinoid, is a naphthoic acid derivative with retinoid-like properties. The chemical name for adapalene is (6-[3-(1-adamantyl)-4-methoxyphenyl]-2- naphthoic acid). It has the following structural formula:
Adapalene:
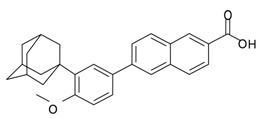
Molecular formula: C28H28O3 Molecular weight: 412.5
Benzoyl Peroxide is a highly lipophilic oxidizing agent that localizes in both bacterial and keratinocyte cell membranes. The chemical name for benzoyl peroxide is dibenzoyl peroxide. It has the following structural formula:
Benzoyl Peroxide:
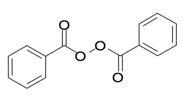
Molecular formula: C14H10O4 Molecular weight: 242.23
EPIDUO gel contains the following inactive ingredients: acrylamide/sodium acryloyldimethyltaurate copolymer, docusate sodium, edetate disodium, glycerin, isohexadecane, poloxamer 124, polysorbate 80, propylene glycol, purified water, and sorbitan oleate.
Sources