Exforge Hct Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Exforge HCT (amlodipine, valsartan, hydrochlorothiazide) is indicated for the treatment of hypertension.
This fixed combination drug is not indicated for the initial therapy of hypertension [see Dosage and Administration (2)].
History
There is currently no drug history available for this drug.
Other Information
Exforge HCT is a fixed combination of amlodipine, valsartan and hydrochlorothiazide.
Exforge HCT contains the besylate salt of amlodipine, a dihydropyridine calcium channel blocker (CCB). Amlodipine besylate, USP is a white to pale yellow crystalline powder, slightly soluble in water and sparingly soluble in ethanol. Amlodipine besylate’s chemical name is 3-Ethyl 5-methyl (±)-2-[(2-aminoethoxy)methyl]-4-(o-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate, monobenzenesulfonate ; its structural formula is
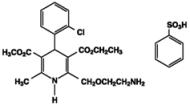
Its empirical formula is C20H25ClN2O5•C6H6O3S and its molecular weight is 567.1.
Valsartan, USP is a nonpeptide, orally active, and specific angiotensin II antagonist acting on the AT1 receptor subtype. Valsartan is a white to practically white fine powder, soluble in ethanol and methanol and slightly soluble in water. Valsartan’s chemical name is N-(1-oxopentyl)-N-[[2′-(1H-tetrazol-5-yl) [1,1′-biphenyl]-4-yl]methyl]-L-valine; its structural formula is
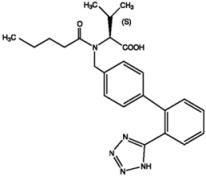
Its empirical formula is C24H29N5O3 and its molecular weight is 435.5.
Hydrochlorothiazide, USP is a white, or practically white, practically odorless, crystalline powder. It is slightly soluble in water; freely soluble in sodium hydroxide solution, in n-butylamine, and in dimethylformamide; sparingly soluble in methanol; and insoluble in ether, in chloroform, and in dilute mineral acids. Hydrochlorothiazide is chemically described as 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7- sulfonamide 1,1-dioxide.
Hydrochlorothiazide is a thiazide diuretic. Its empirical formula is C7H8ClN3O4S2, its molecular weight is 297.73, and its structural formula is
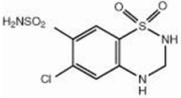
Exforge HCT film-coated tablets are formulated in five strengths for oral administration with a combination of amlodipine besylate, valsartan and hydrochlorothiazide, providing for the following available combinations: 5/160/12.5 mg, 10/160/12.5 mg, 5/160/25 mg, 10/160/25 mg and 10/320/25 mg amlodipine besylate/valsartan/hydrochlorothiazide. The inactive ingredients for all strengths of the tablets include microcrystalline cellulose; crospovidone; colloidal anhydrous silica; magnesium stearate; hypromellose, macrogol 4000 and talc. Additionally, the 5/160/12.5 mg strength contains titanium dioxide; the 10/160/12.5 mg strength contains titanium dioxide and yellow and red iron oxides; the 5/160/25 mg strength contains titanium dioxide and yellow iron oxide and the 10/160/25 mg and 10/320/25 mg strengths both contain yellow iron oxide.
Sources