FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Eyewash Station Additive Concentrate Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
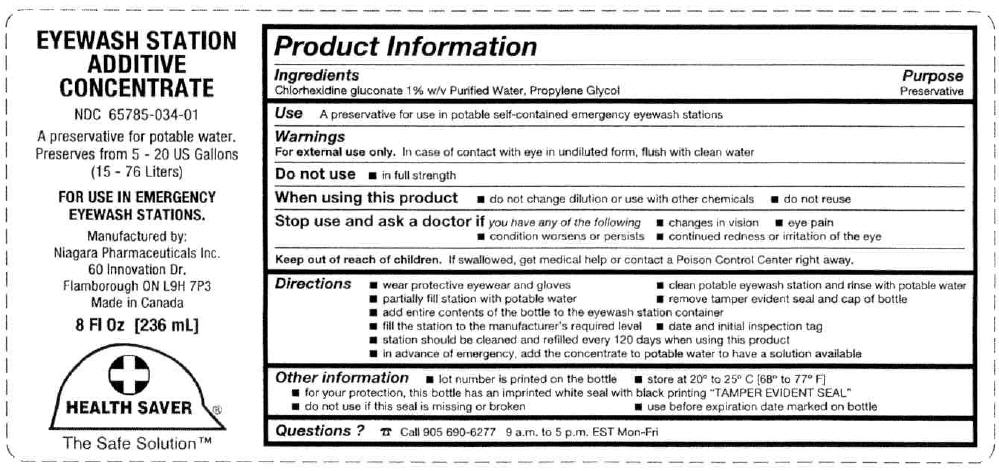
For external use only. In case of contact with eye in undiluted form, flush with clean water
- in full strength
- do not change dilution or use with other chemicals
- do not reuse
Stop use and ask a doctor if you have any of the following
- changes in vision
- eye pain
- condition worsens or persists
- continued redness or irritation of the eye
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
- in full strength
Stop use and ask a doctor if you have any of the following
- changes in vision
- eye pain
- condition worsens or persists
- continued redness or irritation of the eye
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
A preservative for use in potable self-contained emergency eyewash stations
History
There is currently no drug history available for this drug.
Other Information
There are no additional details available for this product.
Sources
Eyewash Station Additive Concentrate Manufacturers
-
Niagara Pharmaceuticals Inc.
![Eyewash Station Additive Concentrate (Chlorhexidine Gluconate And Propylene Glycol) Liquid [Niagara Pharmaceuticals Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Eyewash Station Additive Concentrate | Niagara Pharmaceuticals Inc.
![Eyewash Station Additive Concentrate (Chlorhexidine Gluconate And Propylene Glycol) Liquid [Niagara Pharmaceuticals Inc.] Eyewash Station Additive Concentrate (Chlorhexidine Gluconate And Propylene Glycol) Liquid [Niagara Pharmaceuticals Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
wear protective eyewear and gloves clean potable eyewash station and rinse with potable water partially fill station with potable water remove tamper evident seal and cap of bottle add entire contents of the bottle to the eyewash station container fill the station to the manufacturer's required level date and initial inspection tag station should be cleaned and refilled every 120 days when using this product in advance of emergency, add the concentrate to potable water to have a solution available
Login To Your Free Account