FDA records indicate that there are no current recalls for this drug.
EzriCare Artifical Tears Voluntary Recall
EzriCare Artificial Tears are special eye drops that help make your eyes feel better when they are dry. They have a special ingredient called carboxymethylcellulose sodium. This ingredient is also found in other eye drops. EzriCare Artificial Tears have 10 mg of this special ingredient in 1 ml of the eye drop solution. You can buy these eye drops at the store without a doctor's note because they are over-the-counter. People use these eye drops to help with dry eyes. They can also help protect your eyes from getting more irritated. If your eyes feel uncomfortable because of the sun or wind, these eye drops might help too. Your doctor might tell you to use EzriCare Artificial Tears for other reasons not listed on the bottle. To use the eye drops, just put one or two drops in your eye when you need it.
Are you a medical professional?
Trending Topics
EzriCare Artificial Tears Recall
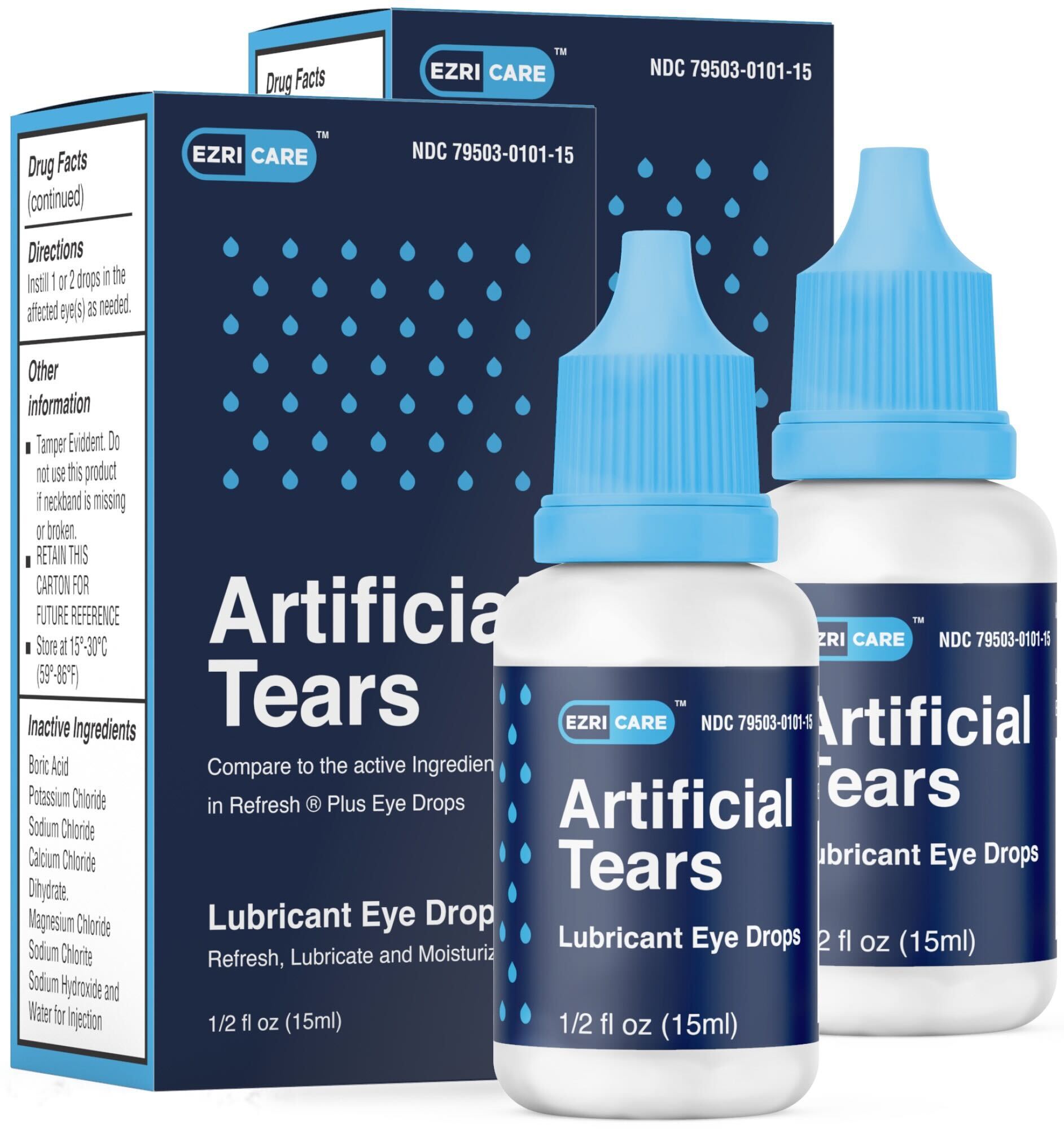
Artificial Tears (carboxymethylcellulose sodium) Lubricant Eye Drops, 10 mg in 1 mL, ½ fl oz (15 ml) bottle are used as a protectant against further irritation or to relieve dryness of the eye for the temporary relief of discomfort due to minor irritations of the eye, or to exposure to wind or sun. The product is packaged in a bottle with a safety seal and are placed in a carton box Ezricare NDC 79503-0101-15, UPC 3 79503 10115 7; Delsam Pharma’s NDC 72570-121-15, UPC 3 72570 12115 8. It can be identified by the photos below. The product was distributed Nationwide in the USA over the Internet. Global Pharma Healthcare is notifying the distributors of this product, Aru Pharma Inc. and Delsam Pharma and is requesting that wholesalers, retailers and customers who have the recalled product should stop use. Consumers with questions regarding this recall can contact the distributors: Aru Pharma/Ezricare, LLC by phone: 1-516-715-5181 or by e-mail: arupharmainc@yahoo.com from Monday to Friday, 11am to 4pm EST; or DELSAM Pharma LLC by phone: 1-866-826-1306 or by e-mail: delsampharma@yahoo.com from Monday to Friday from 11am to 4pm EST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using these over-the-counter drug products. Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online
- Regular Mail or Fax: Download form or call 1- 800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
Questions & Answers
Side Effects & Adverse Reactions
Use of contaminated artificial tears can result in the risk of eye infections that could result in blindness.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-consumers-not-purchase-or-use-ezricare-artificial-tears-due-potential-contamination FDA is warning consumers and health care practitioners not to purchase and to immediately stop using EzriCare Artificial Tears or Delsam Pharma’s Artificial Tears due to potential bacterial contamination. Using contaminated artificial tears increases risk of eye infections that could result in blindness or death. Patients who have signs or symptoms of an eye infection should talk to their health care provider or seek medical care immediately. These are over-the-counter products, manufactured by Global Pharma Healthcare Private Limited, intended to be sterile. Global Pharma initiated a voluntary recall at the consumer level of all unexpired lots of EzriCare Artificial Tears and Delsam Pharma’s Artificial Tears. FDA recommended this recall due to the company’s current good manufacturing practice (CGMP) violations, including lack of appropriate microbial testing, formulation issues (the company manufactures and distributes ophthalmic drugs in multi-use bottles, without an adequate preservative), and lack of proper controls concerning tamper-evident packaging. FDA is collaborating with the Centers for Disease Control and Prevention (CDC) and state and local health departments to investigate a multistate outbreak involving a rare, extensively drug-resistant strain of Pseudomonas aeruginosa bacteria. As of January 31, 2023, CDC identified 55 patients in 12 states with infections that have been linked by epidemiologic and laboratory evidence to use of EzriCare Artificial Tears. Associated adverse events include hospitalization, one death with bloodstream infection, and permanent vision loss from eye infections. CDC issued an alert recommending consumers stop using EzriCare Artificial Tears pending additional guidance from CDC and FDA. FDA also placed Global Pharma Healthcare Private Limited on import alert for providing an inadequate response to a records request and for not complying with CGMP requirements. The import alert prevents these products from entering the United States. FDA encourages health care professionals and patients to report adverse events or quality problems with any medicine to FDA’s MedWatch Adverse Event Reporting program:
- Complete and submit the report online at Medwatch; or
- Download and complete the form, then submit it via fax at 1-800-FDA-0178.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
People use EzriCare Artificial Tears to relieve eye dryness. It may also help protect eyes from further irritation. These artificial tears may also treat discomfort from minor irritations of the eye from exposure to sun or wind. Your doctor may recommend EzriCare Artificial Tears for other uses not on the label. According to dosage information, people should use one or two drops in the affected eye as needed.
History
There is currently no drug history available for this drug.
Other Information
There is currently no other information available for this drug.