FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Flarex Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
FOR TOPICAL OPHTHALMIC USE ONLY. NOT FOR INJECTION. Use in the treatment of herpes simplex infection requires great caution. Prolonged use may result in glaucoma, damage to the optic nerve, defect in visual acuity and visual field, cataract formation and/or may aid in the establishment of secondary ocular infections from pathogens due to suppression of host response. Acute purulent infections of the eye may be masked or exacerbated by presence of steroid medication. In those diseases causing thinning of the cornea or sclera, perforation has been known to occur with chronic use of topical steroids. It is advisable that the intraocular pressure be checked frequently.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
FLAREX® (fluorometholone acetate ophthalmic suspension) is indicated for use in the treatment of steroid responsive inflammatory conditions of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the eye.
History
There is currently no drug history available for this drug.
Other Information
FLAREX® (fluorometholone acetate ophthalmic suspension) is a corticosteroid prepared as a sterile topical ophthalmic suspension. The active ingredient, fluorometholone acetate, is a white to creamy white powder with an empirical formula of C24H31FO5 and a molecular weight of 418.5. Its chemical name is 9-fluoro-11β, 17-dihydroxy-6α-methylpregna-1, 4-diene-3, 20-dione 17-acetate. The chemical structure of Fluorometholone Acetate is presented below:

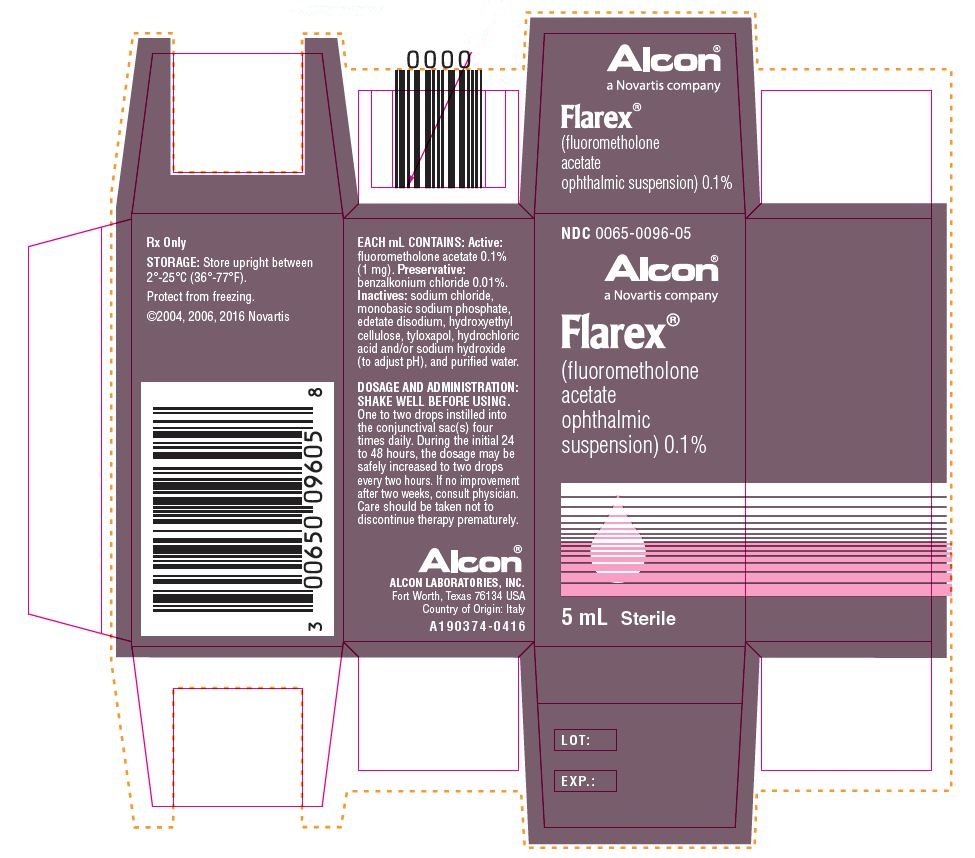
Each mL contains: Active: fluorometholone acetate 1 mg (0.1%). Preservative: benzalkonium chloride 0.01%. Inactives: sodium chloride, monobasic sodium phosphate, edetate disodium, hydroxyethyl cellulose, tyloxapol, hydrochloric acid and/or sodium hydroxide (to adjust pH), and purified water. The pH of the suspension is approximately 7.3, with an osmolality of approximately 300 mOsm/kg.
Sources
Flarex Manufacturers
-
Alcon Laboratories, Inc.
![Flarex (Fluorometholone Acetate) Suspension [Alcon Laboratories, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Flarex | Alcon Laboratories, Inc.
![Flarex (Fluorometholone Acetate) Suspension [Alcon Laboratories, Inc.] Flarex (Fluorometholone Acetate) Suspension [Alcon Laboratories, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Shake Well Before Using. One to two drops instilled into the conjunctival sac(s) four times daily. During the initial 24 to 48 hours the dosage may be safely increased to two drops every two hours. If no improvement after two weeks, consult physician. Care should be taken not to discontinue therapy prematurely.
Login To Your Free Account