FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Fragmin Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
FRAGMIN Injection is indicated for the prophylaxis of ischemic complications in unstable angina and non-Q-wave myocardial infarction, when concurrently administered with aspirin therapy [see Clinical Studies (14.1)].
FRAGMIN is also indicated for the prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE):
- In patients undergoing hip replacement surgery [see Clinical Studies (14.2)];
- In patients undergoing abdominal surgery who are at risk for thromboembolic complications [see Clinical Studies (14.3)];
- In medical patients who are at risk for thromboembolic complications due to severely restricted mobility during acute illness [see Clinical Studies (14.4)].
FRAGMIN is also indicated for the extended treatment of symptomatic venous thromboembolism (VTE) (proximal DVT and/or PE), to reduce the recurrence of VTE in patients with cancer. In these patients, the FRAGMIN therapy begins with the initial VTE treatment and continues for six months [see Clinical Studies (14.5)].
Limitations of Use
FRAGMIN is not indicated for the acute treatment of VTE.
History
There is currently no drug history available for this drug.
Other Information
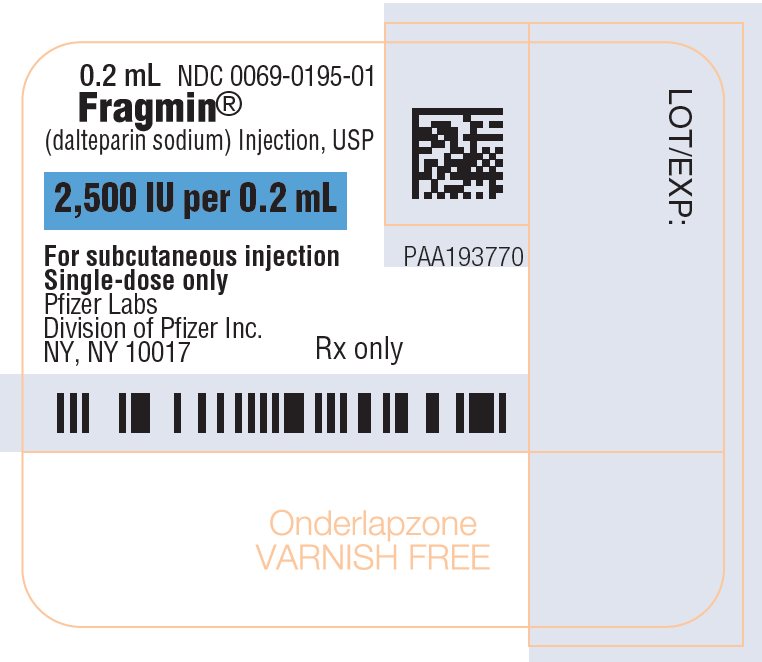
FRAGMIN Injection (dalteparin sodium injection) is a sterile, low molecular weight heparin. It is available in single-dose, prefilled syringes preassembled with a needle guard device, and multiple-dose vials. With reference to the W.H.O. First International Low Molecular Weight Heparin Reference Standard, each syringe contains either 2,500, 5,000, 7,500, 10,000, 12,500, 15,000 or 18,000 anti-Factor Xa international units (IU), equivalent to 16, 32, 48, 64, 80, 96 or 115.2 mg dalteparin sodium, respectively. Each multiple-dose vial contains either 10,000 or 25,000 anti-Factor Xa IU per 1 mL (equivalent to 64 or 160 mg dalteparin sodium, respectively), for a total of 95,000 anti-Factor Xa IU per vial.
Each prefilled syringe also contains Water for Injection and sodium chloride, when required, to maintain physiologic ionic strength. The prefilled syringes are preservative-free. Each multiple-dose vial also contains Water for Injection and 14 mg of benzyl alcohol per mL as a preservative. The pH of both formulations is 5.0 to 7.5 [see Dosage and Administration (2) and How Supplied (16)].
Dalteparin sodium is produced through controlled nitrous acid depolymerization of sodium heparin from porcine intestinal mucosa followed by a chromatographic purification process. It is composed of strongly acidic sulfated polysaccharide chains (oligosaccharide, containing 2,5-anhydro-D-mannitol residues as end groups) with an average molecular weight of 5,000 and about 90% of the material within the range 2,000–9,000. The molecular weight distribution is:
| < | 3000 daltons | 3.0–15% |
| 3,000 to 8,000 daltons | 65.0–78.0% | |
| > | 8,000 daltons | 14.0–26.0% |
STRUCTURAL FORMULA
Sources
Fragmin Manufacturers
-
Pfizer Laboratories Div Pfizer Inc
![Fragmin (Dalteparin Sodium) Injection [Pfizer Laboratories Div Pfizer Inc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Fragmin | Pfizer Laboratories Div Pfizer Inc
![Fragmin (Dalteparin Sodium) Injection [Pfizer Laboratories Div Pfizer Inc] Fragmin (Dalteparin Sodium) Injection [Pfizer Laboratories Div Pfizer Inc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
FRAGMIN is administered by subcutaneous injection. It must not be administered by intramuscular injection.
FRAGMIN Injection should not be mixed with other injections or infusions unless specific compatibility data are available that support such mixing.
Routine coagulation tests such as Prothrombin Time (PT) and Activated Partial Thromboplastin Time (APTT) are relatively insensitive measures of FRAGMIN activity and, therefore, unsuitable for monitoring the anticoagulant effect of FRAGMIN [see Warnings and Precautions (5)].
2.1 Adult DosageProphylaxis of Ischemic Complications in Unstable Angina and Non-Q-Wave Myocardial Infarction: In patients with unstable angina or non-Q-wave myocardial infarction, the recommended dose of FRAGMIN Injection is 120 IU/kg of body weight, but not more than 10,000 IU, subcutaneously every 12 hours with concurrent oral aspirin (75 to 165 mg once daily) therapy. Treatment should be continued until the patient is clinically stabilized. The usual duration of administration is 5 to 8 days. Concurrent aspirin therapy is recommended except when contraindicated.
Table 1 lists the volume of FRAGMIN in mL (based on the 3.8 mL multiple-dose vial 25,000 IU/ mL) and quantity of Fragmin in IU, to be administered for a range of patient weights.
Table 1 Quantity and Volume of FRAGMIN to be Administered by Patient Weight Patient weight (lb) < 110 110 to 131 132 to 153 154 to 175 176 to 197 ≥198 Patient weight (kg) < 50 50 to 59 60 to 69 70 to 79 80 to 89 ≥90 Quantity of FRAGMIN (IU) 5,500 IU 6,500 IU 7,500 IU 9,000 IU 10,000 IU 10,000 IU Volume of FRAGMIN (mL) 95,000 IU / 3.8 mL 0.22 0.26 0.30 0.36 0.40 0.40Prophylaxis of Venous Thromboembolism Following Hip Replacement Surgery: Table 2 presents the dosing options for patients undergoing hip replacement surgery. The usual duration of administration is 5 to 10 days after surgery; up to 14 days of treatment with FRAGMIN have been well tolerated in clinical trials.
Table 2 Dosing Options for Patients Undergoing Hip Replacement Surgery Timing of First Dose of FRAGMIN Dose of FRAGMIN to be Given Subcutaneously 10 to 14 Hours Before Surgery Within 2 Hours Before Surgery 4 to 8 Hours After Surgery* Postoperative Period† * Or later, if hemostasis has not been achieved. † Up to 14 days of treatment was well tolerated in controlled clinical trials, where the usual duration of treatment was 5 to 10 days postoperatively. ‡ Allow a minimum of 6 hours between this dose and the dose to be given on Postoperative Day 1. Adjust the timing of the dose on Postoperative Day 1 accordingly. § Allow approximately 24 hours between doses. Postoperative Start --- --- 2,500 IU‡ 5,000 IU once daily Preoperative Start - Day of
Surgery --- 2,500 IU 2,500 IU‡ 5,000 IU once daily Preoperative Start - Evening Before Surgery§ 5,000 IU --- 5,000 IU 5,000 IU once dailyAbdominal Surgery: In patients undergoing abdominal surgery with a risk of thromboembolic complications, the recommended dose of FRAGMIN is 2,500 IU administered by subcutaneous injection once daily, starting 1 to 2 hours prior to surgery and repeated once daily postoperatively. The usual duration of administration is 5 to 10 days.
In patients undergoing abdominal surgery associated with a high risk of thromboembolic complications, such as malignant disorder, the recommended dose of FRAGMIN is 5,000 IU subcutaneously the evening before surgery, then once daily postoperatively. The usual duration of administration is 5 to 10 days. Alternatively, in patients with malignancy, 2,500 IU of FRAGMIN can be administered subcutaneously 1 to 2 hours before surgery followed by 2,500 IU subcutaneously 12 hours later, and then 5,000 IU once daily postoperatively. The usual duration of administration is 5 to 10 days.
Medical Patients During Acute Illness: In medical patients with severely restricted mobility during acute illness, the recommended dose of FRAGMIN is 5,000 IU administered by subcutaneous injection once daily. In clinical trials, the usual duration of administration was 12 to 14 days.
Extended Treatment of Symptomatic Venous Thromboembolism in Patients with Cancer: In patients with cancer and symptomatic venous thromboembolism, the recommended dosing of FRAGMIN is as follows: for the first 30 days of treatment administer FRAGMIN 200 IU/kg total body weight subcutaneously once daily. The total daily dose should not exceed 18,000 IU. Table 3 lists the dose of FRAGMIN to be administered once daily during the first month for a range of patient weights
Month 1
Table 3 Dose of FRAGMIN to be Administered Subcutaneously by Patient Weight during the First Month Body Weight (lbs) Body Weight (kg) FRAGMIN Dose (IU) (prefilled syringe) once daily ≤ 124 ≤ 56 10,000 125 to 150 57 to 68 12,500 151 to 181 69 to 82 15,000 182 to 216 83 to 98 18,000 ≥ 217 ≥ 99 18,000Months 2 to 6
Administer FRAGMIN at a dose of approximately 150 IU/kg, subcutaneously once daily during Months 2 through 6. The total daily dose should not exceed 18,000 IU. Table 4 lists the dose of FRAGMIN to be administered once daily for a range of patient weights during months 2–6.
Table 4 Dose of FRAGMIN to be Administered Subcutaneously by Patient Weight during Months 2–6 Body Weight (lbs) Body Weight (kg) FRAGMIN Dose (IU) (prefilled syringe) once daily ≤ 124 ≤ 56 7,500 125 to 150 57 to 68 10,000 151 to 181 69 to 82 12,500 182 to 216 83 to 98 15,000 ≥ 217 ≥ 99 18,000Safety and efficacy beyond six months have not been evaluated in patients with cancer and acute symptomatic VTE [see Warnings and Precaution (5) and Adverse Reactions (6.1)].
2.2 Dose Reductions for Thrombocytopenia in Patients with Cancer and Acute Symptomatic VTEIn patients receiving FRAGMIN who experience platelet counts between 50,000 and 100,000/mm3, reduce the daily dose of FRAGMIN by 2,500 IU until the platelet count recovers to ≥100,000/mm3. In patients receiving FRAGMIN who experience platelet counts < 50,000/mm3, discontinue FRAGMIN until the platelet count recovers above 50,000/mm3.
2.3 Dose Reductions for Renal Insufficiency in Extended Treatment of Acute Symptomatic Venous Thromboembolism in Patients with CancerIn patients with severely impaired renal function (CrCl < 30 mL/min), monitor anti-Xa levels to determine the appropriate FRAGMIN dose. Target anti-Xa range is 0.5–1.5 IU/mL. When monitoring anti-Xa in these patients, perform sampling 4–6 hrs after FRAGMIN dosing and only after the patient has received 3–4 doses.
2.4 AdministrationSubcutaneous injection technique: Patients should be sitting or lying down and FRAGMIN administered by deep subcutaneous injection. FRAGMIN may be injected in a U-shape area around the navel, the upper outer side of the thigh or the upper outer quadrangle of the buttock. The injection site should be varied daily. When the area around the navel or the thigh is used, using the thumb and forefinger, you must lift up a fold of skin while giving the injection. The entire length of the needle should be inserted at a 45 to 90 degree angle.
Inspect FRAGMIN prefilled syringes and vials visually for particulate matter and discoloration prior to administration
After first penetration of the rubber stopper, store the multiple-dose vials at room temperature for up to 2 weeks. Discard any unused solution after 2 weeks.
Instructions for using the prefilled single-dose syringes preassembled with needle guard devices
Fixed dose syringes: To ensure delivery of the full dose, do not expel the air bubble from the prefilled syringe before injection. Hold the syringe assembly by the open sides of the device. Remove the needle shield. Insert the needle into the injection area as instructed above. Depress the plunger of the syringe while holding the finger flange until the entire dose has been given. The needle guard will not be activated unless the entire dose has been given. Remove needle from the patient. Let go of the plunger and allow syringe to move up inside the device until the entire needle is guarded. Discard the syringe assembly in approved containers.
Graduated syringes: Hold the syringe assembly by the open sides of the device. Remove the needle shield. With the needle pointing up, prepare the syringe by expelling the air bubble and then continuing to push the plunger to the desired dose or volume, discarding the extra solution in an appropriate manner. Insert the needle into the injection area as instructed above. Depress the plunger of the syringe while holding the finger flange until the entire dose remaining in the syringe has been given. The needle guard will not be activated unless the entire dose has been given. Remove needle from the patient. Let go of the plunger and allow syringe to move up inside the device until the entire needle is guarded. Discard the syringe assembly in approved containers.
-
Pfizer, Inc.
![Fragmin (Dalteparin Sodium) Injection, Solution [Pfizer, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Fragmin | Pfizer, Inc.
![Fragmin (Dalteparin Sodium) Injection, Solution [Pfizer, Inc.] Fragmin (Dalteparin Sodium) Injection, Solution [Pfizer, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Prophylaxis of Ischemic Complications in Unstable Angina and Non-Q-Wave Myocardial InfarctionIn patients with unstable angina or non-Q-wave myocardial infarction, the recommended dose of FRAGMIN Injection is 120 IU/kg of body weight, but not more than 10,000 IU, subcutaneously (s.c.) every 12 hours with concurrent oral aspirin (75 to 165 mg once daily) therapy. Treatment should be continued until the patient is clinically stabilized. The usual duration of administration is 5 to 8 days. Concurrent aspirin therapy is recommended except when contraindicated.
Table 13 lists the volume of FRAGMIN, based on the 9.5 mL multiple-dose vial (10,000 IU/mL), to be administered for a range of patient weights.
Table 13 Volume of FRAGMIN to be Administered by Patient Weight, Based on 9.5 mL Vial (10,000 IU/mL) Patient
weight (lb) < 110 110 to 131 132 to 153 154 to 175 176 to 197 ≥198 Patient
weight (kg) < 50 50 to 59 60 to 69 70 to 79 80 to 89 ≥90 Volume of
FRAGMIN (mL) 0.55 0.65 0.75 0.90 1.0 1.0 Prophylaxis of Venous Thromboembolism Following Hip Replacement SurgeryTable 14 presents the dosing options for patients undergoing hip replacement surgery. The usual duration of administration is 5 to 10 days after surgery; up to 14 days of treatment with FRAGMIN have been well tolerated in clinical trials.
Table 14 Dosing Options for Patients Undergoing Hip Replacement Surgery Dose of FRAGMIN to be Given Subcutaneously Timing of
First Dose
of FRAGMIN 10 to 14 Hours
Before
Surgery Within 2 Hours
Before
Surgery 4 to 8 Hours
After
Surgery* Postoperative
Period† * Or later, if hemostasis has not been achieved. † Up to 14 days of treatment was well tolerated in controlled clinical trials, where the usual duration of treatment was 5 to 10 days postoperatively. ‡ Allow a minimum of 6 hours between this dose and the dose to be given on Postoperative Day 1. Adjust the timing of the dose on Postoperative Day 1 accordingly. § Allow approximately 24 hours between doses. Postoperative
Start --- --- 2500 IU‡ 5000 IU once daily Preoperative
Start - Day of
Surgery --- 2500 IU 2500 IU‡ 5000 IU once daily Preoperative
Start - Evening
Before Surgery§ 5000 IU --- 5000 IU 5000 IU once daily Prophylaxis of Venous Thromboembolism Following Abdominal SurgeryIn patients undergoing abdominal surgery with a risk of thromboembolic complications, the recommended dose of FRAGMIN is 2500 IU administered by s.c. injection once daily, starting 1 to 2 hours prior to surgery and repeated once daily postoperatively. The usual duration of administration is 5 to 10 days.
In patients undergoing abdominal surgery associated with a high risk of thromboembolic complications, such as malignant disorder, the recommended dose of FRAGMIN is 5000 IU s.c. the evening before surgery, then once daily postoperatively. The usual duration of administration is 5 to 10 days. Alternatively, in patients with malignancy, 2500 IU of FRAGMIN can be administered s.c. 1 to 2 hours before surgery followed by 2500 IU s.c. 12 hours later, and then 5000 IU once daily postoperatively. The usual duration of administration is 5 to 10 days.
Dosage adjustment and routine monitoring of coagulation parameters are not required if the dosage and administration recommendations specified above are followed.
Medical Patients with Severely Restricted Mobility During Acute IllnessIn medical patients with severely restricted mobility during acute illness, the recommended dose of FRAGMIN is 5000 IU administered by s.c. injection once daily. In clinical trials, the usual duration of administration was 12 to 14 days.
Extended Treatment of Symptomatic Venous Thromboembolism in Patients with CancerIn patients with cancer and symptomatic venous thromboembolism, the recommended dosing of FRAGMIN is as follows: for the first 30 days of treatment administer FRAGMIN 200 IU/kg total body weight subcutaneously (s.c.) once daily. The total daily dose should not exceed 18,000 IU. Table 15 lists the dose of FRAGMIN to be administered once daily during the first month for a range of patient weights.
Month 1 Table 15 Dose of FRAGMIN to be Administered Subcutaneously by Patient Weight during the First Month Body Weight (lbs) Body Weight (kg) FRAGMIN Dose (IU)
(prefilled syringe) once daily ≤ 124 ≤ 56 10,000 125 to 150 57 to 68 12,500 151 to 181 69 to 82 15,000 182 to 216 83 to 98 18,000 ≥ 217 ≥ 99 18,000 Months 2 to 6Administer FRAGMIN at a dose of approximately 150 IU/kg, s.c. once daily during Months 2 through 6. The total daily dose should not exceed 18,000 IU. Table 16 lists the dose of FRAGMIN to be administered once daily for a range of patient weights during months 2–6.
Table 16 Dose of FRAGMIN to be Administered Subcutaneously by Patient Weight during Months 2–6 Body Weight (lbs) Body Weight (kg) FRAGMIN Dose (IU)
(prefilled syringe) once daily ≤ 124 ≤ 56 7,500 125 to 150 57 to 68 10,000 151 to 181 69 to 82 12,500 182 to 216 83 to 98 15,000 ≥ 217 ≥ 99 18,000Safety and efficacy beyond six months have not been evaluated in patients with cancer and acute symptomatic VTE (see WARNINGS, Thrombocytopenia and ADVERSE REACTIONS, Patients with Cancer and Acute Symptomatic VTE).
Dose reductions for thrombocytopenia in patients with cancer and acute symptomatic VTEIn patients receiving FRAGMIN who experience platelet counts between 50,000 and 100,000/mm3, reduce the daily dose of FRAGMIN by 2,500 IU until the platelet count recovers to ≥100,000/mm3. In patients receiving FRAGMIN who experience platelet counts < 50,000/mm3, FRAGMIN should be discontinued until the platelet count recovers above 50,000/mm3.
Dose reductions for renal insufficiency in extended treatment of acute symptomatic venous thromboembolism in patients with cancerIn patients with severely impaired renal function (CrCl < 30 mL/min), monitoring for anti-Xa levels is recommended to determine the appropriate FRAGMIN dose. Target anti-Xa range is 0.5–1.5 IU/mL. When monitoring anti-Xa in these patients, sampling should be performed 4–6 hrs after FRAGMIN dosing and only after the patient has received 3–4 doses.
AdministrationFRAGMIN is administered by subcutaneous injection. It must not be administered by intramuscular injection.
Subcutaneous injection technique: Patients should be sitting or lying down and FRAGMIN administered by deep s.c. injection. FRAGMIN may be injected in a U-shape area around the navel, the upper outer side of the thigh or the upper outer quadrangle of the buttock. The injection site should be varied daily. When the area around the navel or the thigh is used, using the thumb and forefinger, you must lift up a fold of skin while giving the injection. The entire length of the needle should be inserted at a 45 to 90 degree angle.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
After first penetration of the rubber stopper, store the multiple-dose vials at room temperature for up to 2 weeks. Discard any unused solution after 2 weeks.
Instructions for using the prefilled single-dose syringes preassembled with needle guard devices Fixed dose syringesTo ensure delivery of the full dose, do not expel the air bubble from the prefilled syringe before injection. Hold the syringe assembly by the open sides of the device. Remove the needle shield. Insert the needle into the injection area as instructed above. Depress the plunger of the syringe while holding the finger flange until the entire dose has been given. The needle guard will not be activated unless the entire dose has been given. Remove needle from the patient. Let go of the plunger and allow syringe to move up inside the device until the entire needle is guarded. Discard the syringe assembly in approved containers.
Graduated syringesHold the syringe assembly by the open sides of the device. Remove the needle shield. With the needle pointing up, prepare the syringe by expelling the air bubble and then continuing to push the plunger to the desired dose or volume, discarding the extra solution in an appropriate manner. Insert the needle into the injection area as instructed above. Depress the plunger of the syringe while holding the finger flange until the entire dose remaining in the syringe has been given. The needle guard will not be activated unless the entire dose has been given. Remove needle from the patient. Let go of the plunger and allow syringe to move up inside the device until the entire needle is guarded. Discard the syringe assembly in approved containers.
-
Eisai Inc.
![Fragmin (Dalteparin Sodium) Injection [Eisai Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Fragmin | Eisai Inc.
![Fragmin (Dalteparin Sodium) Injection [Eisai Inc.] Fragmin (Dalteparin Sodium) Injection [Eisai Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
FRAGMIN is administered by subcutaneous injection. It must not be administered by intramuscular injection.
FRAGMIN Injection should not be mixed with other injections or infusions unless specific compatibility data are available that support such mixing.
Routine coagulation tests such as Prothrombin Time (PT) and Activated Partial Thromboplastin Time (APTT) are relatively insensitive measures of FRAGMIN activity and, therefore, unsuitable for monitoring the anticoagulant effect of FRAGMIN. [see Warnings and Precautions (5)].
2.1 Adult DosageProphylaxis of Ischemic Complications in Unstable Angina and Non-Q-Wave Myocardial Infarction: In patients with unstable angina or non-Q-wave myocardial infarction, the recommended dose of FRAGMIN Injection is 120 IU/kg of body weight, but not more than 10,000 IU, subcutaneously every 12 hours with concurrent oral aspirin (75 to 165 mg once daily) therapy. Treatment should be continued until the patient is clinically stabilized. The usual duration of administration is 5 to 8 days. Concurrent aspirin therapy is recommended except when contraindicated.
Table 1 lists the volume of FRAGMIN, based on the 9.5 mL multiple-dose vial (10,000 IU/mL), to be administered for a range of patient weights.
Table 1 Volume of FRAGMIN to be Administered by Patient Weight, Based on
9.5 mL Vial (10,000 IU/mL) Patient
weight (lb) < 110 110 to 131 132 to 153 154 to 175 176 to 197 ≥ 198 Patient
weight (kg) < 50 50 to 59 60 to 69 70 to 79 80 to 89 ≥ 90 Volume of
FRAGMIN (mL) 0.55 0.65 0.75 0.90 1.0 1.0Prophylaxis of Venous Thromboembolism Following Hip Replacement Surgery: Table 2 presents the dosing options for patients undergoing hip replacement surgery. The usual duration of administration is 5 to 10 days after surgery; up to 14 days of treatment with FRAGMIN have been well tolerated in clinical trials.
Table 2 Dosing Options for Patients Undergoing Hip Replacement Surgery Timing of First
Dose
of FRAGMIN Dose of FRAGMIN to be Given Subcutaneously 10 to 14 Hours
Before Surgery Within 2 Hours
Before Surgery 4 to 8 Hours
After Surgery1 Postoperative
Period2 Postoperative
Start --- --- 2500 IU3 5000 IU once daily Preoperative
Start - Day of
Surgery --- 2500 IU 2500 IU3 5000 IU once daily Preoperative
Start - Evening
Before Surgery4 5000 IU --- 5000 IU 5000 IU once daily1 Or later, if hemostasis has not been achieved.
2 Up to 14 days of treatment was well tolerated in controlled clinical trials, where the usual duration of treatment was 5 to 10 days postoperatively.
3 Allow a minimum of 6 hours between this dose and the dose to be given on Postoperative Day 1. Adjust the timing of the dose on Postoperative Day 1 accordingly.
4 Allow approximately 24 hours between doses.Abdominal Surgery: In patients undergoing abdominal surgery with a risk of thromboembolic complications, the recommended dose of FRAGMIN is 2500 IU administered by subcutaneous injection once daily, starting 1 to 2 hours prior to surgery and repeated once daily postoperatively. The usual duration of administration is 5 to 10 days.
In patients undergoing abdominal surgery associated with a high risk of thromboembolic complications, such as malignant disorder, the recommended dose of FRAGMIN is 5000 IU subcutaneously the evening before surgery, then once daily postoperatively. The usual duration of administration is 5 to 10 days. Alternatively, in patients with malignancy, 2500 IU of FRAGMIN can be administered subcutaneously 1 to 2 hours before surgery followed by 2500 IU subcutaneously 12 hours later, and then 5000 IU once daily postoperatively. The usual duration of administration is 5 to 10 days.
Medical Patients During Acute Illness: In medical patients with severely restricted mobility during acute illness, the recommended dose of FRAGMIN is 5000 IU administered by subcutaneous injection once daily. In clinical trials, the usual duration of administration was 12 to 14 days.
Extended Treatment of Symptomatic Venous Thromboembolism in Patients with Cancer: In patients with cancer and symptomatic venous thromboembolism, the recommended dosing of FRAGMIN is as follows: for the first 30 days of treatment administer FRAGMIN 200 IU/kg total body weight subcutaneously once daily. The total daily dose should not exceed 18,000 IU. Table 3 lists the dose of FRAGMIN to be administered once daily during the first month for a range of patient weights.
Month 1
Table 3 Dose of FRAGMIN to be Administered Subcutaneously by Patient
Weight during the First Month Body Weight (lbs) Body Weight (kg) FRAGMIN Dose (IU)
(prefilled syringe)
once daily ≤ 124 ≤ 56 10,000 125 to 150 57 to 68 12,500 151 to 181 69 to 82 15,000 182 to 216 83 to 98 18,000 ≥ 217 ≥ 99 18,000Months 2 to 6
Administer FRAGMIN at a dose of approximately 150 IU/kg, subcutaneously once daily during Months 2 through 6. The total daily dose should not exceed 18,000 IU. Table 4 lists the dose of FRAGMIN to be administered once daily for a range of patient weights during months 2-6.
Table 4 Dose of FRAGMIN to be Administered Subcutaneously by Patient
Weight during Months 2-6 Body Weight (lbs) Body Weight
(kg) FRAGMIN Dose (IU)
(prefilled syringe)
once daily ≤ 124 ≤ 56 7,500 125 to 150 57 to 68 10,000 151 to 181 69 to 82 12,500 182 to 216 83 to 98 15,000 ≥ 217 ≥ 99 18,000Safety and efficacy beyond six months have not been evaluated in patients with cancer and acute symptomatic VTE [see Warnings and Precaution (5) and Adverse Reactions (6.1)].
2.2 Dose Reductions for Thrombocytopenia in Patients with Cancer and Acute Symptomatic VTEIn patients receiving FRAGMIN who experience platelet counts between 50,000 and 100,000/mm3, reduce the daily dose of FRAGMIN by 2,500 IU until the platelet count recovers to ≥ 100,000/mm3. In patients receiving FRAGMIN who experience platelet counts < 50,000/mm3, discontinue FRAGMIN until the platelet count recovers above 50,000/mm3.
2.3 Dose Reductions for Renal Insufficiency in Extended Treatment of Acute Symptomatic Venous Thromboembolism in Patients with CancerIn patients with severely impaired renal function (CrCl < 30 mL/min), monitor anti-Xa levels to determine the appropriate FRAGMIN dose. Target anti-Xa range is 0.5-1.5 IU/mL. When monitoring anti-Xa in these patients, perform sampling 4-6 hrs after FRAGMIN dosing and only after the patient has received 3-4 doses.
2.4 AdministrationSubcutaneous injection technique: Patients should be sitting or lying down and FRAGMIN administered by deep subcutaneous injection. FRAGMIN may be injected in a U-shape area around the navel, the upper outer side of the thigh or the upper outer quadrangle of the buttock. The injection site should be varied daily. When the area around the navel or the thigh is used, using the thumb and forefinger, you must lift up a fold of skin while giving the injection. The entire length of the needle should be inserted at a 45 to 90 degree angle.
Inspect FRAGMIN prefilled syringes and vials visually for particulate matter and discoloration prior to administration
After first penetration of the rubber stopper, store the multiple-dose vials at room temperature for up to 2 weeks. Discard any unused solution after 2 weeks.
Instructions for using the prefilled single-dose syringes preassembled with needle guard devicesFixed dose syringes: To ensure delivery of the full dose, do not expel the air bubble from the prefilled syringe before injection. Hold the syringe assembly by the open sides of the device. Remove the needle shield. Insert the needle into the injection area as instructed above. Depress the plunger of the syringe while holding the finger flange until the entire dose has been given. The needle guard will not be activated unless the entire dose has been given. Remove needle from the patient. Let go of the plunger and allow syringe to move up inside the device until the entire needle is guarded. Discard the syringe assembly in approved containers.
Graduated syringes: Hold the syringe assembly by the open sides of the device. Remove the needle shield. With the needle pointing up, prepare the syringe by expelling the air bubble and then continuing to push the plunger to the desired dose or volume, discarding the extra solution in an appropriate manner. Insert the needle into the injection area as instructed above. Depress the plunger of the syringe while holding the finger flange until the entire dose remaining in the syringe has been given. The needle guard will not be activated unless the entire dose has been given. Remove needle from the patient. Let go of the plunger and allow syringe to move up inside the device until the entire needle is guarded. Discard the syringe assembly in approved containers.
Login To Your Free Account


![Fragmin (Dalteparin Sodium) Injection, Solution [Pfizer, Inc.]](http://recallguide.cwdevelopsp.com/wp-content/themes/recallguide/assets/img/drug-image-placeholder.jpg)
![Fragmin (Dalteparin Sodium) Injection [Eisai Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=c5cd4a8e-14c0-440b-b453-9f3d3250c951&name=fragmin-10.jpg)