FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Ilaris Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
ILARIS (canakinumab) is an interleukin-1β blocker indicated for the treatment of Cryopyrin-Associated Periodic Syndromes (CAPS), in adults and children 4 years of age and older including:
- Familial Cold Autoinflammatory Syndrome (FCAS)
- Muckle-Wells Syndrome (MWS)
ILARIS is indicated for the treatment of active Systemic Juvenile Idiopathic Arthritis (SJIA) in patients aged 2 years and older.
History
There is currently no drug history available for this drug.
Other Information
Canakinumab is a recombinant, human anti-human-IL-1β monoclonal antibody that belongs to the IgG1/κ isotype subclass. It is expressed in a murine Sp2/0-Ag14 cell line and comprised of two 447- (or 448-) residue heavy chains and two 214-residue light chains, with a molecular mass of 145157 Daltons when deglycosylated. Both heavy chains of canakinumab contain oligosaccharide chains linked to the protein backbone at asparagine 298 (Asn 298).
The biological activity of canakinumab is measured by comparing its inhibition of IL-1β-dependent expression of the reporter gene luciferase to that of a canakinumab internal reference standard, using a stably transfected cell line.
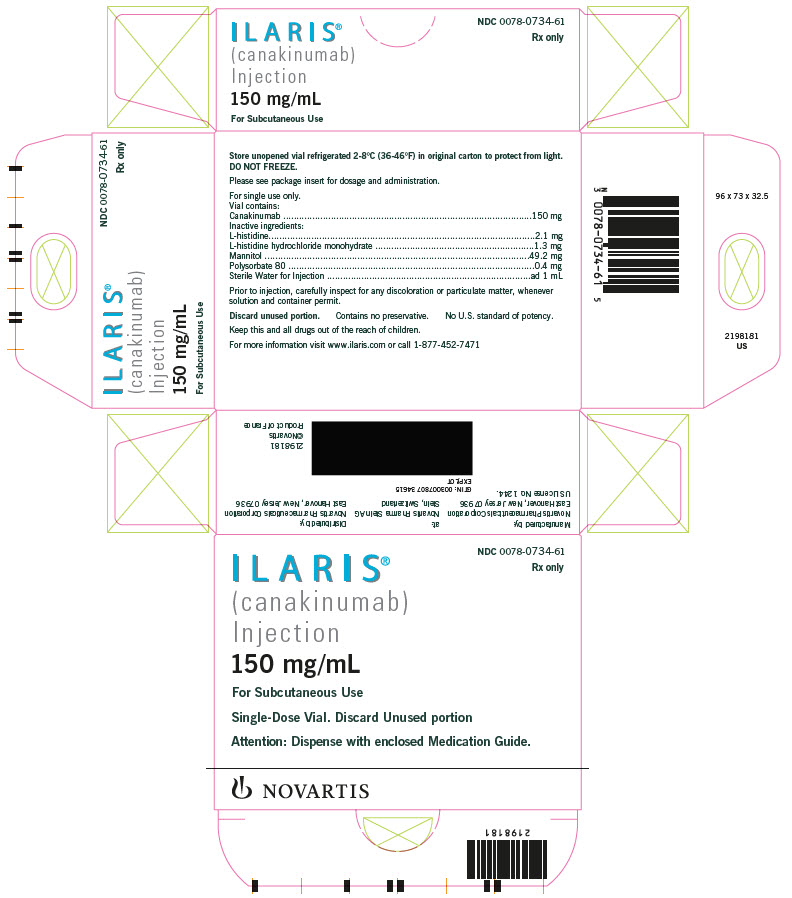
ILARIS is supplied in a sterile, single-use, colorless, 6 mL glass vial with coated stopper and aluminum flip-off cap. Each vial contains 180 mg of canakinumab as a white, preservative-free, lyophilized powder. Reconstitution with 1 mL of preservative-free Sterile Water for Injection is required prior to subcutaneous administration of the drug. The reconstituted canakinumab is a 150 mg/mL solution essentially free of particulates, clear to slightly opalescent, and is colorless or may have a slightly brownish-yellow tint. A volume of up to 1 mL can be withdrawn for delivery of 150 mg/mL canakinumab for subcutaneous administration. Each reconstituted vial contains 180 mg canakinumab, sucrose, L-histidine, L-histidine HCL monohydrate, polysorbate 80 and Sterile Water for Injection. No preservatives are present.
Sources
Ilaris Manufacturers
-
Novartis Pharmaceuticals Corporation
![Ilaris (Canakinumab) Injection, Powder, Lyophilized, For Solution [Novartis Pharmaceuticals Corporation]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Ilaris | Novartis Pharmaceuticals Corporation
![Ilaris (Canakinumab) Injection, Powder, Lyophilized, For Solution [Novartis Pharmaceuticals Corporation] Ilaris (Canakinumab) Injection, Powder, Lyophilized, For Solution [Novartis Pharmaceuticals Corporation]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
2.1 General Dosing InformationINJECTION FOR SUBCUTANEOUS USE ONLY.
2.2 Cryopyrin-Associated Periodic Syndromes (CAPS)The recommended dose of ILARIS is 150 mg for CAPS patients with body weight greater than 40 kg. For CAPS patients with body weight greater than or equal to 15 kg and less than or equal to 40 kg, the recommended dose is 2 mg/kg. For children 15 to 40 kg with an inadequate response, the dose can be increased to 3 mg/kg.
ILARIS is administered every eight weeks as a single dose via subcutaneous injection.
2.3 Systemic Juvenile Idiopathic Arthritis (SJIA)The recommended dose of ILARIS for SJIA patients with a body weight greater than or equal to 7.5 kg is 4 mg/kg (with a maximum of 300 mg) administered every 4 weeks via subcutaneous injection.
2.4 Four Steps for Preparation and AdministrationSTEP 1: Using aseptic technique, reconstitute each vial of ILARIS by slowly injecting 1 mL of preservative-free Sterile Water for Injection with a 1 mL syringe and an 18 gauge x 2” needle.
STEP 2: Swirl the vial slowly at an angle of about 45° for approximately 1 minute and allow to stand for 5 minutes. Do not shake. Then gently turn the vial upside down and back again ten times. Avoid touching the rubber stopper with your fingers.
STEP 3: Allow to stand for about 15 minutes at room temperature to obtain a clear solution. The reconstituted solution has a final concentration of 150 mg/mL. Do not shake. Do not use if particulate matter is present in the solution. Tap the side of the vial to remove any residual liquid from the stopper. The reconstituted solution should be essentially free from particulates, and clear to opalescent. The solution should be colorless or may have a slight brownish-yellow tint. If the solution has a distinctly brown discoloration it should not be used. If not used within 60 minutes of reconstitution, the solution should be stored in the refrigerator at 2°C to 8°C (36°F to 46°F) and used within 4 hours. Slight foaming of the product upon reconstitution is not unusual.
STEP 4: Using a sterile syringe and needle carefully withdraw the required volume depending on the dose to be administered (0.2 mL to 1 mL) and subcutaneously inject using a 27 gauge x 0.5” needle.
Injection into scar tissue should be avoided as this may result in insufficient exposure to ILARIS.
ILARIS 180 mg powder for solution for injection is supplied in a single-use vial. Any unused product or waste material should be disposed of in accordance with local requirements.
Login To Your Free Account