Imitrex Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
IMITREX Tablets should only be used where a clear diagnosis of migraine headache has been established.
Risk of Myocardial Ischemia and/or Infarction and Other Adverse Cardiac Events
Sumatriptan should not be given to patients with documented ischemic or vasospastic coronary artery disease (CAD) (see CONTRAINDICATIONS). It is strongly recommended that sumatriptan not be given to patients in whom unrecognized CAD is predicted by the presence of risk factors (e.g., hypertension, hypercholesterolemia, smoker, obesity, diabetes, strong family history of CAD, female with surgical or physiological menopause, male over 40 years of age) unless a cardiovascular evaluation provides satisfactory clinical evidence that the patient is reasonably free of coronary artery and ischemic myocardial disease or other significant underlying cardiovascular disease. The sensitivity of cardiac diagnostic procedures to detect cardiovascular disease or predisposition to coronary artery vasospasm is modest, at best. If, during the cardiovascular evaluation, the patient’s medical history or electrocardiographic investigations reveal findings indicative of, or consistent with, coronary artery vasospasm or myocardial ischemia, sumatriptan should not be administered (see CONTRAINDICATIONS).
For patients with risk factors predictive of CAD, who are determined to have a satisfactory cardiovascular evaluation, it is strongly recommended that administration of the first dose of sumatriptan tablets take place in the setting of a physician’s office or similar medically staffed and equipped facility unless the patient has previously received sumatriptan. Because cardiac ischemia can occur in the absence of clinical symptoms, consideration should be given to obtaining on the first occasion of use an electrocardiogram (ECG) during the interval immediately following IMITREX Tablets in these patients with risk factors.
It is recommended that patients who are intermittent long-term users of sumatriptan and who have or acquire risk factors predictive of CAD, as described above, undergo periodic interval cardiovascular evaluation as they continue to use sumatriptan.
The systematic approach described above is intended to reduce the likelihood that patients with unrecognized cardiovascular disease will be inadvertently exposed to sumatriptan.
Drug-Associated Cardiac Events and Fatalities
Serious adverse cardiac events, including acute myocardial infarction, life-threatening disturbances of cardiac rhythm, and death have been reported within a few hours following the administration of IMITREX® (sumatriptan succinate) Injection or IMITREX Tablets. Considering the extent of use of sumatriptan in patients with migraine, the incidence of these events is extremely low.
The fact that sumatriptan can cause coronary vasospasm, that some of these events have occurred in patients with no prior cardiac disease history and with documented absence of CAD, and the close proximity of the events to sumatriptan use support the conclusion that some of these cases were caused by the drug. In many cases, however, where there has been known underlying coronary artery disease, the relationship is uncertain.
Premarketing Experience With Sumatriptan
Of 6,348 patients with migraine who participated in premarketing controlled and uncontrolled clinical trials of oral sumatriptan, 2 experienced clinical adverse events shortly after receiving oral sumatriptan that may have reflected coronary vasospasm. Neither of these adverse events was associated with a serious clinical outcome.
Among the more than 1,900 patients with migraine who participated in premarketing controlled clinical trials of subcutaneous sumatriptan, there were 8 patients who sustained clinical events during or shortly after receiving sumatriptan that may have reflected coronary artery vasospasm. Six of these 8 patients had ECG changes consistent with transient ischemia, but without accompanying clinical symptoms or signs. Of these 8 patients, 4 had either findings suggestive of CAD or risk factors predictive of CAD prior to study enrollment.
Among approximately 4,000 patients with migraine who participated in premarketing controlled and uncontrolled clinical trials of sumatriptan nasal spray, 1 patient experienced an asymptomatic subendocardial infarction possibly subsequent to a coronary vasospastic event.
Postmarketing Experience With Sumatriptan
Serious cardiovascular events, some resulting in death, have been reported in association with the use of IMITREX Injection or IMITREX Tablets. The uncontrolled nature of postmarketing surveillance, however, makes it impossible to determine definitively the proportion of the reported cases that were actually caused by sumatriptan or to reliably assess causation in individual cases. On clinical grounds, the longer the latency between the administration of IMITREX and the onset of the clinical event, the less likely the association is to be causative. Accordingly, interest has focused on events beginning within 1 hour of the administration of IMITREX.
Cardiac events that have been observed to have onset within 1 hour of sumatriptan administration include: coronary artery vasospasm, transient ischemia, myocardial infarction, ventricular tachycardia and ventricular fibrillation, cardiac arrest, and death.
Some of these events occurred in patients who had no findings of CAD and appear to represent consequences of coronary artery vasospasm. However, among domestic reports of serious cardiac events within 1 hour of sumatriptan administration, almost all of the patients had risk factors predictive of CAD and the presence of significant underlying CAD was established in most cases (see CONTRAINDICATIONS).
Drug-Associated Cerebrovascular Events and Fatalities
Cerebral hemorrhage, subarachnoid hemorrhage, stroke, and other cerebrovascular events have been reported in patients treated with oral or subcutaneous sumatriptan, and some have resulted in fatalities. The relationship of sumatriptan to these events is uncertain. In a number of cases, it appears possible that the cerebrovascular events were primary, sumatriptan having been administered in the incorrect belief that the symptoms experienced were a consequence of migraine when they were not. As with other acute migraine therapies, before treating headaches in patients not previously diagnosed as migraineurs, and in migraineurs who present with atypical symptoms, care should be taken to exclude other potentially serious neurological conditions. It should also be noted that patients with migraine may be at increased risk of certain cerebrovascular events (e.g., cerebrovascular accident, transient ischemic attack).
Other Vasospasm-Related Events
Sumatriptan may cause vasospastic reactions other than coronary artery vasospasm. Both peripheral vascular ischemia and colonic ischemia with abdominal pain and bloody diarrhea have been reported. Very rare reports of transient and permanent blindness and significant partial vision loss have been reported with the use of sumatriptan. Visual disorders may also be part of a migraine attack.
Serotonin Syndrome
The development of a potentially life-threatening serotonin syndrome may occur with triptans, including treatment with IMITREX, particularly during combined use with selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs). If concomitant treatment with sumatriptan and an SSRI (e.g., fluoxetine, paroxetine, sertraline, fluvoxamine, citalopram, escitalopram) or SNRI (e.g., venlafaxine, duloxetine) is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases. Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
Increase in Blood Pressure
Significant elevation in blood pressure, including hypertensive crisis, has been reported on rare occasions in patients with and without a history of hypertension. Sumatriptan is contraindicated in patients with uncontrolled hypertension (see CONTRAINDICATIONS). Sumatriptan should be administered with caution to patients with controlled hypertension as transient increases in blood pressure and peripheral vascular resistance have been observed in a small proportion of patients.
Concomitant Drug Use
In patients taking MAO-A inhibitors, sumatriptan plasma levels attained after treatment with recommended doses are 7-fold higher following oral administration than those obtained under other conditions. Accordingly, the coadministration of IMITREX Tablets and an MAO-A inhibitor is contraindicated (see CLINICAL PHARMACOLOGY and CONTRAINDICATIONS).
Hypersensitivity
Hypersensitivity (anaphylaxis/anaphylactoid) reactions have occurred on rare occasions in patients receiving sumatriptan. Such reactions can be life threatening or fatal. In general, hypersensitivity reactions to drugs are more likely to occur in individuals with a history of sensitivity to multiple allergens (see CONTRAINDICATIONS).
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
IMITREX Tablets are indicated for the acute treatment of migraine attacks with or without aura in adults.
IMITREX Tablets are not intended for the prophylactic therapy of migraine or for use in the management of hemiplegic or basilar migraine (see CONTRAINDICATIONS). Safety and effectiveness of IMITREX Tablets have not been established for cluster headache, which is present in an older, predominantly male population.
History
There is currently no drug history available for this drug.
Other Information
IMITREX Tablets contain sumatriptan (as the succinate), a selective 5-hydroxytryptamine1 receptor subtype agonist. Sumatriptan succinate is chemically designated as 3-[2-(dimethylamino)ethyl]-N-methyl-indole-5-methanesulfonamide succinate (1:1), and it has the following structure:
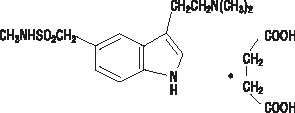
The empirical formula is C
14H
21N
3O
2S•C
4H
6O
4, representing a molecular weight of 413.5. Sumatriptan succinate is a white to off-white powder that is readily soluble in water and in saline. Each IMITREX Tablet for oral administration contains 35, 70, or 140 mg of sumatriptan succinate equivalent to 25, 50, or 100 mg of sumatriptan, respectively. Each tablet also contains the inactive ingredients croscarmellose sodium, dibasic calcium phosphate, magnesium stearate, microcrystalline cellulose, and sodium bicarbonate. Each 100-mg tablet also contains hypromellose, iron oxide, titanium dioxide, and triacetin.
Sources





 The empirical formula is C
The empirical formula is C![Imitrex (Sumatriptan Succinate) Injection [Physicians Total Care, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=413cb899-509f-40cb-b898-c44af9611ce7&name=3180.jpg)
![Imitrex (Sumatriptan Succinate) Tablet, Film Coated [Lake Erie Medical & Surgical Supply Dba Quality Care Products Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=7624d1f1-5c03-4a34-a69a-402ae3c49ee8&name=Imitrex100mgGSK.jpg)
![Imitrex (Sumatriptan Succinate) Tablet, Film Coated [Physicians Total Care, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=040abcc1-cc1c-4f00-b302-36070611f7b2&name=3777.jpg)
![Imitrex (Sumatriptan Succinate) Tablet, Film Coated [Glaxosmithkline Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=70ffae5e-ca7f-4ba2-be11-2d50fc9450ad&name=10mgBottleLabels-01.jpg)
![Imitrex (Sumatriptan) Spray [Glaxosmithkline Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=cc11b09d-e6cc-4cf8-1495-5ac64d5aae5f&name=imitrex-nasalspray-spl-graphic-10.jpg)
![Imitrex (Sumatriptan Succinate) Injection [Glaxosmithkline Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=47fc0880-5982-4dcc-a8c7-81b891fde8ce&name=Illusionctn.jpg)