Levocarnitine Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
None.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Levocarnitine is indicated in the treatment of primary systemic carnitine deficiency. In the reported cases, the clinical presentation consisted of recurrent episodes of Reye-like encephalopathy, hypoketotic hypoglycemia, and/or cardiomyopathy. Associated symptoms included hypotonia, muscle weakness and failure to thrive. A diagnosis of primary carnitine deficiency requires that serum, red cell and/or tissue carnitine levels be low and that the patient does not have a primary defect in fatty acid or organic acid oxidation (see CLINICAL PHARMACOLOGY). In some patients, particularly those presenting with cardiomyopathy, carnitine supplementation rapidly alleviated signs and symptoms. Treatment should include, in addition to carnitine, supportive and other therapy as indicated by the condition of the patient.
Levocarnitine is also indicated for acute and chronic treatment of patients with an inborn error of metabolism which results in a secondary carnitine deficiency.
History
There is currently no drug history available for this drug.
Other Information
Levocarnitine is a carrier molecule in the transport of long-chain fatty acids across the inner mitochondrial membrane.
The chemical name of levocarnitine is 3-carboxy-2(R)-hydroxy-N,N,N-trimethyl-1-propanaminium, inner salt. Levocarnitine is a white crystalline, hygroscopic powder. It is readily soluble in water, hot alcohol, and insoluble in acetone. The specific rotation of levocarnitine is between -29° and -32°. Its chemical structure is:
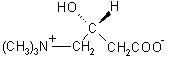
Empirical Formula:C7H15NO3
Molecular Weight: 161.20
Each Levocarnitine Tablet USP contains 330 mg of levocarnitine and the inactive ingredients magnesium stearate, microcrystalline cellulose and povidone.
Sources