Losartan Potassium Manufacturers
-
Direct Rx
-
Mckesson Packaging Services
-
Remedyrepack Inc.
-
Proficient Rx Lp
-
Remedyrepack Inc.
-
Remedyrepack Inc.
-
Direct Rx
-
Legacy Pharmaceutical Packaging
-
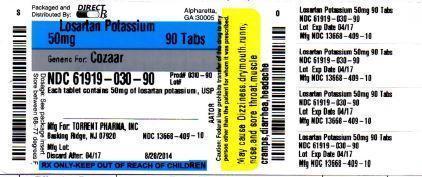
Torrent Pharmaceuticals Limited
-
Endo Pharmaceuticals Inc. Dba Endo Generic Products
-
Remedyrepack Inc.
-
Dispensing Solutions, Inc.
-
Stat Rx Usa Llc
-
Medvantx, Inc.
-
Remedyrepack Inc.
-
Medvantx, Inc.
-
Unit Dose Services
-
Life Line Home Care Services, Inc.
-
Cardinal Health
-
Physicians Total Care, Inc.
-
Legacy Pharmaceutical Packaging
-
Bryant Ranch Prepack
-
Mylan Pharmaceuticals Inc.
-
Northstar Rx Llc
-
Remedyrepack Inc.
-
Preferred Pharmaceuticals, Inc.
-
Watson Laboratories, Inc.
-
Remedyrepack Inc.
-
Remedyrepack Inc.
-
Mckesson Contract Packaging
-
Cadila Healthcare Limited
-
Qualitest Pharmaceuticals
-
Remedyrepack Inc.
-
Remedyrepack Inc.
-
Golden State Medical Supply, Inc.
-
Preferred Pharmaceuticals, Inc
-
Remedyrepack Inc.
-
Preferred Pharmaceuticals, Inc.
-
Macleods Pharmaceuticals Limited
-
Clinical Solutions Wholesale
-
Lake Erie Medical Dba Quality Care Products Llc
-
Legacy Pharmaceutical Packaging
-
St Marys Medical Park Pharmacy
-
Pack Pharmaceuticals, Llc
-
Clinical Solutions Wholesale
-
Pd-rx Pharmaceuticals, Inc.
-
Pd-rx Pharmaceuticals, Inc.
-
Pd-rx Pharmaceuticals, Inc.
-
Unit Dose Services
-
Unit Dose Services
-
Mckesson Packaging Services A Business Unit Of Mckesson Corporation
-
Ncs Healthcare Of Ky, Inc Dba Vangard Labs
-
Teva Pharmaceuticals Usa Inc
-
Aurobindo Pharma Limited
-
Sandoz Inc
-
Apotex Corp
-
Remedyrepack Inc.
-
St Marys Medical Park Pharmacy
-
State Of Florida Doh Central Pharmacy
-
Lupin Pharmaceuticals, Inc.
-
Jubilant Cadista Pharmaceuticals Inc.
-
Major Pharmaceuticals
-
Legacy Pharmaceutical Packaging
-
Readymeds
-
Carilion Materials Management
-
Carilion Materials Management
-
Roxane Laboratories, Inc.
-
Micro Labs Limited
-
Virtus Pharmaceuticals Llc
-
Avpak
-
State Of Florida Doh Central Pharmacy
-
Camber Pharmaceuticals, Inc.
-
Medsource Pharmaceuticals
-
Remedyrepack Inc.
Login To Your Free Account




![Losartan Potassium Tablet, Film Coated [Mckesson Packaging Services]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=aefb7fb6-c89e-4804-a637-3555293bac3e&name=losartanpotasiumtabs-figure-08.jpg)
![Losartan Potassium Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=52901bc7-6193-447a-90cb-0ae66b028e43&name=MM7.jpg)
![Losartan Potassium Tablet, Film Coated [Proficient Rx Lp]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=28e7fd03-42bc-44fd-846d-41df31788746&name=25-90.jpg)
![Losartan Potassium Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=9ac2d9f0-0e13-43e8-86be-c2fbf23f3a6a&name=MM7.jpg)
![Losartan Potassium Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=3a38c9ca-d134-4dd6-8d4f-5c082c1a5ef2&name=MM7.jpg)
![Losartan Potassium Tablet [Direct Rx]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=d6ba182e-b768-4913-8688-7f29676b05c9&name=label.jpg)
![Losartan Potassium Tablet [Legacy Pharmaceutical Packaging]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=8064bbeb-14a3-411c-8e86-e17ac9a7aa05&name=senna-s-01.jpg)
![Losartan Potassium Tablet [Torrent Pharmaceuticals Limited]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=4c2347db-79a3-4279-87f8-9fa91e6d00e3&name=losartanpotassiumusp-figure-08.jpg)
![Losartan Potassium Tablet, Film Coated [Endo Pharmaceuticals Inc. Dba Endo Generic Products]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=e3dffeff-e499-4b88-88ec-a968a35e1e18&name=100-90s.jpg)
![Losartan Potassium Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=aad4de9c-164c-49b2-930e-03f8026588e1&name=MM6.jpg)
![Losartan Potassium Tablet, Film Coated [Dispensing Solutions, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=42e16ddf-4eb1-447e-9c90-06844f9e1246&name=NDC66336-0665-XX------TEVA.jpg)
![Losartan Potassium Tablet [Stat Rx Usa Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=149f17ea-98b4-46cc-a80b-e56d18c8b266&name=LOSARTAN100mgLABEL.jpg)
![Losartan Potassium Tablet, Film Coated [Medvantx, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=f88c39fa-ed74-42f4-a886-c268daf8f3a6&name=f88c39fa-ed74-42f4-a886-c268daf8f3a6-06.jpg)
![Losartan Potassium Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=13d36ab1-98c8-4672-8632-c6eb4922de40&name=MM6.jpg)
![Losartan Potassium Tablet, Film Coated [Medvantx, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=69605072-f130-4054-95b0-ff3c34554fb9&name=69605072-f130-4054-95b0-ff3c34554fb9-08.jpg)
![Losartan Potassium Tablet [Unit Dose Services]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=d7e2ff3a-7b3d-45ee-bf6f-de79619f64f2&name=uds6664.jpg)
![Losartan Potassium Tablet [Life Line Home Care Services, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=4b0c8bda-2ee3-4e57-8637-aef38410ef21&name=LOSARTAN50MG.jpg)
![Losartan Potassium Tablet, Film Coated [Cardinal Health]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=8574acd5-ad13-424f-bdfe-152c6623b24c&name=8574acd5-ad13-424f-bdfe-152c6623b24c-05.jpg)
![Losartan Potassium Tablet, Film Coated [Physicians Total Care, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=d87866fd-62c9-4b0a-b8a8-e62516cae0a9&name=6104.jpg)
![Losartan Potassium Tablet, Film Coated [Legacy Pharmaceutical Packaging]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=1a72e2bb-a65b-421b-bdbc-0f70d471f117&name=losartan-potassium-tablets---teva-6.jpg)
![Losartan Potassium Tablet, Film Coated [Bryant Ranch Prepack]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=4c1223dc-c3b7-48d1-844a-a03d6698e0b8&name=45191.jpg)
![Losartan Potassium Tablet, Film Coated [Mylan Pharmaceuticals Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=a092eef4-78b8-43b9-80fc-61341c37ea43&name=e8ca8261-8a1b-4661-a1af-f12bc2be52b8-06.jpg)
![Losartan Potassium Tablet, Film Coated [Northstar Rx Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=9251e824-4aad-4a27-bd5b-041b42f68ba5&name=89ac0645-figure-01.jpg)
![Losartan Potassium Tablet, Film Coated [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=d19b346b-932c-4a41-a1be-1a6e6faeafa4&name=MM6.jpg)
![Losartan Potassium Tablet, Film Coated [Preferred Pharmaceuticals, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=1330e8f9-6bda-49f4-ac44-275ec00e780d&name=cerave-wetskin.jpg)
![Losartan Potassium Tablet, Film Coated [Watson Laboratories, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=7f3b370c-ca97-e0a5-dbd2-870d7c7782cf&name=losartan-potassium-tablets-6.jpg)
![Losartan Potassium Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=66547ccc-0bcc-4443-bfb9-d9df010e28e5&name=MM8.jpg)
![Losartan Potassium Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=c1747999-0c77-41ab-814b-dd91bf7170c7&name=MM8.jpg)
![Losartan Potassium Tablet, Film Coated [Mckesson Contract Packaging]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=d1f6fa09-cea1-4086-8c2a-e27e9a409253&name=d1f6fa09-cea1-4086-8c2a-e27e9a409253-06.jpg)
![Losartan Potassium Tablet, Film Coated [Cadila Healthcare Limited]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=9ccd6823-e813-472c-99cd-c6ef25e74bed&name=losartanpotassium(manu)-figure-06.jpg)
![Losartan Potassium Tablet, Film Coated [Qualitest Pharmaceuticals]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=b8e3cb50-26a0-46ba-a1cb-d4e2e20ca882&name=100-90.jpg)
![Losartan Potassium Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=3d0ac192-6f03-4347-b9fd-b454668e986c&name=MM8.jpg)
![Losartan Potassium Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=26ca023d-d1c8-4b55-8a8a-466a263d9ed5&name=MM8.jpg)
![Losartan Potassium Tablet, Film Coated [Golden State Medical Supply, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=c66550cd-4bee-4507-8f2d-2cff68cca8cd&name=2ebd1e08-2500-463a-902c-7fd18ecc2d82-04.jpg)
![Losartan Potassium Tablet, Film Coated [Preferred Pharmaceuticals, Inc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=61b1a335-fca5-4a57-b24f-ae7d081d06ed&name=image-07.jpg)
![Losartan Potassium Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=1ca2341a-6771-4d29-93e7-9a09b86321b5&name=MM8.jpg)
![Losartan Potassium Tablet [Preferred Pharmaceuticals, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=fa8c2931-35a7-4b4b-8be2-eda701b51079&name=image-02.jpg)
![Losartan Potassium Tablet, Film Coated [Macleods Pharmaceuticals Limited]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=a7788c4b-165c-439d-989a-891d2267d897&name=cough-and-cold-hbp-1.jpg)
![Losartan Potassium Tablet, Film Coated [Clinical Solutions Wholesale]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=ea5909d9-0f0d-40eb-912e-5ee30eb233a1&name=Los100Lab00.jpg)
![Losartan Potassium Tablet [Lake Erie Medical Dba Quality Care Products Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=8413a40b-c538-4c46-b6fe-c602c0a66cd0&name=LosartanPotassium100mgLabelTorrent.jpg)
![Losartan Potassium Tablet [Legacy Pharmaceutical Packaging]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=af6d9dce-820a-43e0-af26-9645e20619ec&name=losartan-potassium-tablets-usp---lupin-6.jpg)
![Losartan Potassium Tablet [St Marys Medical Park Pharmacy]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=e1c02c69-d3bf-4153-a89e-a992c3696a20&name=Losartan100mg.jpg)
![Losartan Potassium Tablet, Film Coated Losartan Potassium Tablet, Film Coated [Pack Pharmaceuticals, Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=69c40af8-3710-46bf-bd3d-137fe5eea23e&name=dextroamphetamine-sulfate-extended-release-6.jpg)
![Losartan Potassium Tablet [Clinical Solutions Wholesale]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=4808c239-9938-4ff9-a29a-598dca2ef351&name=childrens-plus-multi-symptom-cold-1.jpg)
![Losartan Potassium Tablet, Film Coated [Pd-rx Pharmaceuticals, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=dbb75191-a16d-49d4-a41d-33914d6a9f08&name=43063477.jpg)
![Losartan Potassium Tablet, Film Coated [Pd-rx Pharmaceuticals, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=b10ef05e-9a96-44d7-bcdb-de977154fb39&name=43063478.jpg)
![Losartan Potassium Tablet, Film Coated [Pd-rx Pharmaceuticals, Inc.]](https://www.recallguide.org/wp-content/themes/bootstrap/assets/img/drug-image-placeholder.jpg)
![Losartan Potassium Tablet [Unit Dose Services]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=442471c7-65eb-454c-a5ff-ac6134cbc8bb&name=50436-6668.jpg)
![Losartan Potassium Tablet [Unit Dose Services]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=3049c71a-a422-4c02-bafb-d876f068911a&name=50436-6669.jpg)
![Losartan Potassium Tablet, Film Coated [Mckesson Packaging Services A Business Unit Of Mckesson Corporation]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=e8979bdc-eae4-4db9-ab70-68e078a9c661&name=4b85bb76-96b1-4854-8946-50611ca48382-07.jpg)
![Losartan Potassium Tablet, Film Coated [Ncs Healthcare Of Ky, Inc Dba Vangard Labs]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=ad03e5cc-c36d-4ff0-9b25-5d13e2909230&name=losartan-potassium-6.jpg)
![Losartan Potassium Tablet, Film Coated [Teva Pharmaceuticals Usa Inc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=f51fd4fa-ea55-487e-b886-ae105dd5a854&name=image-06.jpg)
![Losartan Potassium Tablet, Film Coated [Aurobindo Pharma Limited]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=31366b4b-dfd6-4fda-babe-2cb3d2c14a2b&name=losartan-fig5.jpg)
![Losartan Potassium Tablet, Film Coated [Sandoz Inc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=499df918-9c97-4e13-ab56-0bec5d61d652&name=0d972052-2917-457c-98e1-0aaa55ec070e-06.jpg)
![Losartan Potassium Tablet, Film Coated [Apotex Corp]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=e7f5010b-aad0-4036-b60f-bfa1360facf0&name=zaleplon-capsules-figure-2.jpg)
![Losartan Potassium Tablet [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=5629a1dc-e041-41dd-9028-4aad484581ad&name=MM8.jpg)
![Losartan Potassium Tablet [St Marys Medical Park Pharmacy]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=74f8b602-84b8-4ace-bd0a-a3544790cad5&name=Losartan25mg.jpg)
![Losartan Potassium Tablet [State Of Florida Doh Central Pharmacy]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=02cdce82-4da9-4c34-89d9-43d542a40261&name=LosartanPotassium100mg.jpg)
![Losartan Potassium Tablet [Lupin Pharmaceuticals, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=e7e6da3b-8485-1382-61c9-e9b369018b98&name=image-12.jpg)
![Losartan Potassium Tablets, 25 Mg (Losartan Potassium) Tablet Losartan Potassium Tablets, 50 Mg (Losartan Potassium) Tablet Losartan Potassium Tablets, 100 Mg (Losartan Potassium) Tablet [Jubilant Cadista Pharmaceuticals Inc.]](http://www.recallguide.org/wp-content/themes/recallguide/assets/img/drug-image-placeholder.jpg)
![Losartan Potassium Tablet [Major Pharmaceuticals]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=e21aa8b3-3f6b-4a4f-88ce-8ccf99f1d201&name=losartan-potassium-tablets-6.jpg)
![Losartan Potassium Tablet, Film Coated [Legacy Pharmaceutical Packaging]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=821aabcf-e4a1-4f37-ba00-7ece7fa75e81&name=losartan-potassium-tablets---kroger-6.jpg)
![Losartan Potassium Tablet, Film Coated [Readymeds]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=523930e1-8176-4d72-9f4d-ea8e5f9ecaed&name=photo1.jpg)
![Losartan Potassium Tablet [Carilion Materials Management]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=e43cab61-af7d-4ad3-aed0-130a7dcc35fd&name=68151-0182.jpg)
![Losartan Potassium Tablet, Film Coated [Carilion Materials Management]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=0cdc3669-3b34-4e28-b60e-7b6d58a853db&name=68151-0183.jpg)
![Losartan Potassium Tablet [Roxane Laboratories, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=db36c4f9-a1b7-48ad-b516-b78b224a5ac5&name=image-06.jpg)
![Losartan Potassium Tablet, Film Coated Losartan Potassium (Losartan Potassium ) Tablet, Film Coated [Micro Labs Limited]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=f2f2b6c6-0ab1-483f-b4a2-78dfe3200153&name=losartanpotassium100mglabl.jpg)
![Losartan Potassium Tablet, Film Coated [Virtus Pharmaceuticals Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=242f50bd-efaa-4aef-b155-f395063e2ced&name=losartan-03.jpg)
![Losartan Potassium (Losartan Potassium) Tablet [Avpak]](http://recallguide.cwdevelopsp.com/wp-content/themes/recallguide/assets/img/drug-image-placeholder.jpg)
![Losartan Potassium Tablet, Film Coated [State Of Florida Doh Central Pharmacy]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=eef67f3a-0ac2-4684-be23-d66f5bd2fc05&name=25mgqualitest.jpg)
![Losartan Potassium Tablet, Film Coated [Camber Pharmaceuticals, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=cafd54e8-42a8-474c-8ea8-49ec74c2b0d3&name=losartan100mg30s.jpg)
![Losartan Potassium Tablet, Film Coated [Medsource Pharmaceuticals]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=1fc12db9-6546-509b-e054-00144ff88e88&name=718-60.jpg)
![Losartan Potassium Tablet, Film Coated [Remedyrepack Inc. ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=66ca17b5-ae2d-470b-918d-f847510dd82f&name=MM6.jpg)