Maxidex Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
Prolonged use may result in ocular hypertension and/or glaucoma, with damage to the optic nerve, defects in visual acuity and fields of vision, and posterior subcapsular cataract formation. Prolonged use may suppress the host response and thus increase the hazard of secondary ocular infections. In those diseases causing thinning of the cornea or sclera, perforations have been known to occur with the use of topical corticosteroids. In acute purulent conditions of the eye, corticosteroids may mask infection or enhance existing infection. If these products are used for 10 days or longer, intraocular pressure should be routinely monitored even though it may be difficult in children and uncooperative patients.
Employment of corticosteroid medication in the treatment of herpes simplex other than epithelial herpes simplex keratitis, in which it is contraindicated, requires great caution; periodic slit-lamp microscopy is essential.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Steroid responsive inflammatory conditions of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the globe such as allergic conjunctivitis, acne rosacea, superficial punctuate keratitis, herpes zoster keratitis, iritis, cyclitis, selected infective conjunctivitides when the inherent hazard of steroid use is accepted to obtain an advisable diminution in edema and inflammation; corneal injury from chemical, radiation, or thermal burns, or penetration of foreign bodies.
History
There is currently no drug history available for this drug.
Other Information
MAXIDEX® 0.1% (dexamethasone ophthalmic suspension) is an adrenocortical steroid prepared as a sterile topical ophthalmic suspension. The active ingredient is represented by the chemical structure:
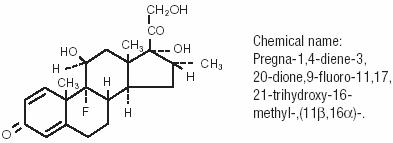
Each mL contains
Active: dexamethasone 0.1%. Preservative: benzalkonium chloride 0.01%. Vehicle: hypromellose 0.5%. Inactives: sodium chloride, dibasic sodium phosphate, polysorbate 80, edetate disodium, citric acid and/or sodium hydroxide (to adjust pH), purified water.
Sources