FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Medaclear Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
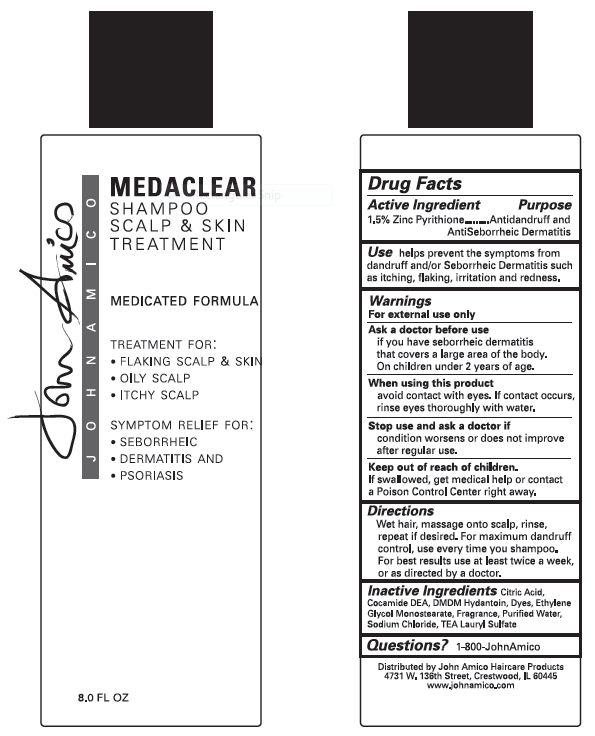
if you have seborrheic dermatitis that covers a large area of the body.
On children under 2 years of age.
avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
condition worsens or does not improve after regular use.
If swallowed, get medical help or contact a Poison Control Center right away.
if you have seborrheic dermatitis that covers a large area of the body.
On children under 2 years of age.
Stop use and ask a doctor ifcondition worsens or does not improve after regular use.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
helps prevent the symptoms from dandruff and/or Seborrheic Dermatitus such as itching, flaking, irritation and redness.
History
There is currently no drug history available for this drug.
Other Information
There are no additional details available for this product.
Sources
Medaclear Manufacturers
-
Amico Educational Concepts, Inc.
Login To Your Free Account