Micardis Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
1.1 Hypertension
MICARDIS is indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive agents [see Clinical Studies (14.1)].
1.2 Cardiovascular Risk Reduction
MICARDIS is indicated for reduction of the risk of myocardial infarction, stroke, or death from cardiovascular causes in patients 55 years of age or older at high risk of developing major cardiovascular events who are unable to take ACE inhibitors.
High risk for cardiovascular events can be evidenced by a history of coronary artery disease, peripheral arterial disease, stroke, transient ischemic attack, or high-risk diabetes (insulin-dependent or non-insulin dependent) with evidence of end-organ damage [see Clinical Studies (14.2)]. MICARDIS can be used in addition to other needed treatment (such as antihypertensive, antiplatelet or lipid-lowering therapy) [see Clinical Studies (14.2)].
Studies of telmisartan in this setting do not exclude that it may not preserve a meaningful fraction of the effect of the ACE inhibitor to which it was compared. Consider using the ACE inhibitor first, and, if it is stopped for cough only, consider re-trying the ACE inhibitor after the cough resolves.
Use of telmisartan with an ACE inhibitor is not recommended [see Warnings and Precautions (5.6)].
History
There is currently no drug history available for this drug.
Other Information
MICARDIS is a non-peptide angiotensin II receptor (type AT1) antagonist.
Telmisartan is chemically described as 4'-[(1,4'-dimethyl-2'-propyl [2,6'-bi-1H-benzimidazol]-1'-yl)methyl]-[1,1'-biphenyl]-2-carboxylic acid. Its empirical formula is C33H30N4O2, its molecular weight is 514.63, and its structural formula is:
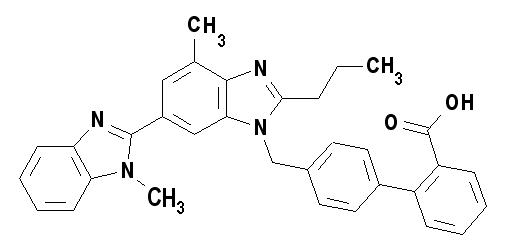
Telmisartan is a white to slightly yellowish solid. It is practically insoluble in water and in the pH range of 3 to 9, sparingly soluble in strong acid (except insoluble in hydrochloric acid), and soluble in strong base.
MICARDIS is available as tablets for oral administration, containing 20 mg, 40 mg or 80 mg of telmisartan. The tablets contain the following inactive ingredients: sodium hydroxide, meglumine, povidone, sorbitol, and magnesium stearate. MICARDIS tablets are hygroscopic and require protection from moisture.
Sources




![Micardis (Telmisartan) Tablet [Lake Erie Medical & Surgical Supply Dba Quality Care Products Llc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=e29933dd-3e4e-4c43-973c-67e7398bf803&name=Micardis80mgBI.jpg)
![Micardis (Telmisartan) Tablet [Boehringer Ingelheim Pharmaceuticals, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=cfb9309f-e0df-4a55-9542-0e869fce05fb&name=carton-003937.jpg)