FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Mitomycin Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
Patients being treated with mitomycin must be observed carefully and frequently during and after therapy.
The use of mitomycin results in a high incidence of bone marrow suppression, particularly thrombocytopenia and leukopenia. Therefore, the following studies should be obtained repeatedly during therapy and for at least eight weeks following therapy: platelet count, white blood cell count, differential, and hemoglobin. The occurrence of a platelet count below 100,000/mm 3 or a WBC below 4,000/mm 3 or a progressive decline in either is an indication to withhold further therapy until blood counts have recovered above these levels.
Patients should be advised of the potential toxicity of this drug, particularly bone marrow suppression. Deaths have been reported due to septicemia as a result of leukopenia due to the drug.
Patients receiving mitomycin should be observed for evidence of renal toxicity. Mitomycin should not be given to patients with a serum creatinine greater than 1.7 mg percent.
Safe use of mitomycin in pregnant women has not been established. Teratological changes have been noted in animal studies. The effect of mitomycin on fertility is unknown.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Mitomycin for Injection is not recommended as single-agent, primary therapy. It has been shown to be useful in the therapy of disseminated adenocarcinoma of the stomach or pancreas in proven combinations with other approved chemotherapeutic agents and as palliative treatment when other modalities have failed. Mitomycin is not recommended to replace appropriate surgery and/or radiotherapy.
History
There is currently no drug history available for this drug.
Other Information
Mitomycin (also known as mitomycin and/or mitomycin-C) is an antibiotic isolated from the broth of Streptomyces caespitosus which has been shown to have antitumor activity. The compound is heat stable, has a high melting point, and is freely soluble in organic solvents.
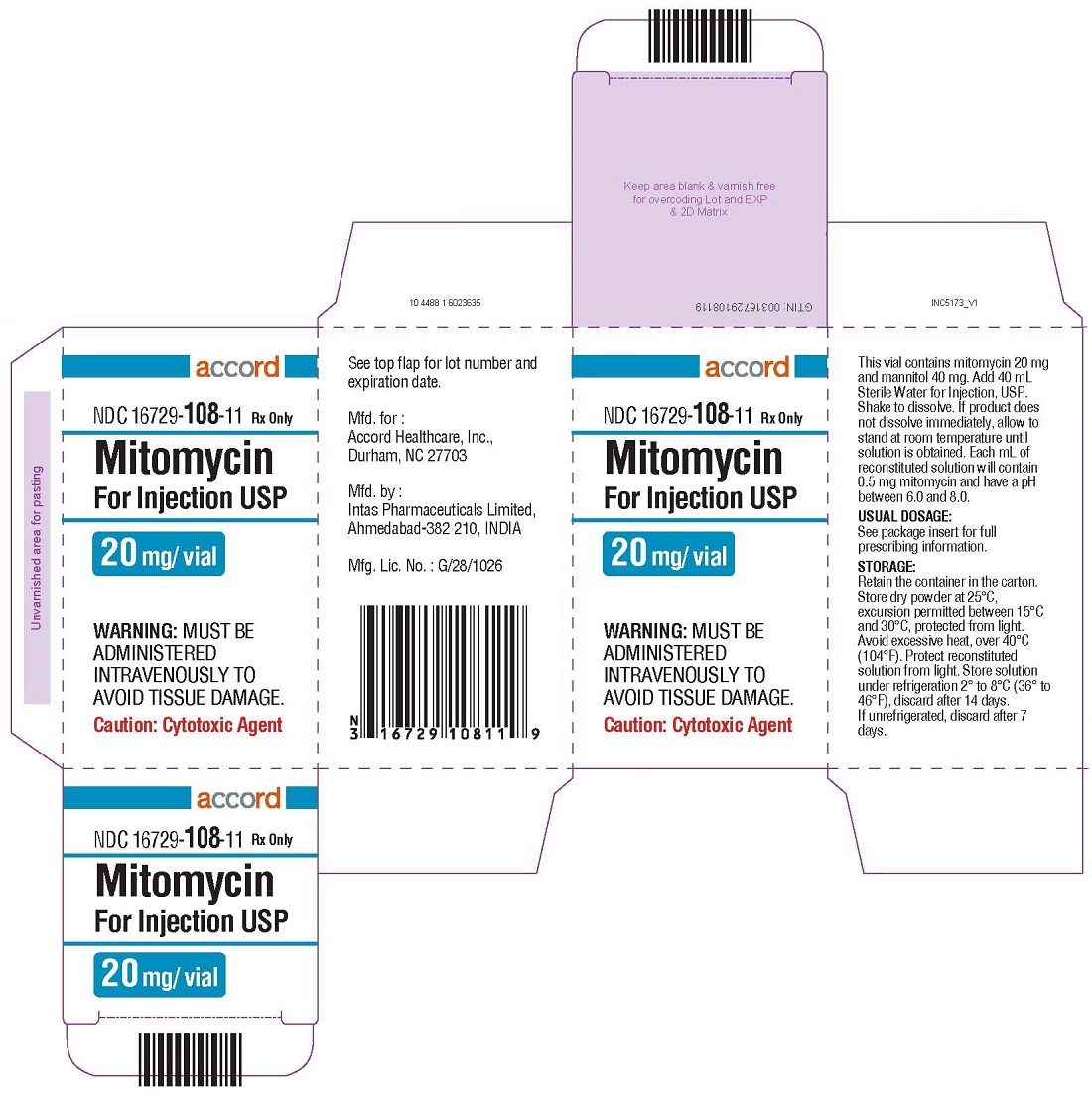
Mitomycin for Injection is a sterile dry mixture of mitomycin and mannitol, which when reconstituted with Sterile Water for Injection provides a solution for intravenous administration. Each vial contains either mitomycin 5 mg and mannitol 10 mg, or mitomycin 20 mg and mannitol 40 mg, or mitomycin 40 mg and mannitol 80 mg. Each mL of reconstituted solution will contain 0.5 mg mitomycin and have a pH between 6.0 and 8.0.
Mitomycin is a blue-violet crystalline powder with the molecular formula of C 15H 18N 4O 5, and a molecular weight of 334.33. Its chemical name is 7-amino-9α-methoxymitosane and it has the following structural formula;

Sources
Mitomycin Manufacturers
-
Accord Healthcare Inc
![Mitomycin Injection, Powder, Lyophilized, For Solution [Accord Healthcare Inc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Mitomycin | Accord Healthcare Inc
![Mitomycin Injection, Powder, Lyophilized, For Solution [Accord Healthcare Inc] Mitomycin Injection, Powder, Lyophilized, For Solution [Accord Healthcare Inc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Mitomycin should be given intravenously only, using care to avoid extravasation of the compound. If extravasation occurs, cellulitis, ulceration, and slough may result.
Each vial contains either mitomycin 5 mg and mannitol 10 mg, mitomycin, 20 mg and mannitol 40 mg or mitomycin 40 mg and mannitol 80 mg. To administer, add Sterile Water for Injection, 10 mL, 40 mL or 80 mL respectively. Shake to dissolve. If product does not dissolve immediately, allow to stand at room temperature until solution is obtained.
After full hematological recovery (see guide to dosage adjustment) from any previous chemotherapy, the following dosage schedule may be used at 6 to 8 week intervals:
20 mg/m 2 intravenously as a single dose via a functioning intravenous catheter.
Because of cumulative myelosuppression, patients should be fully reevaluated after each course of mitomycin, and the dose reduced if the patient has experienced any toxicities. Doses greater than 20 mg/m 2 have not been shown to be more effective, and are more toxic than lower doses.
The following schedule is suggested as a guide to dosage adjustment:
Nadir After Prior Dose Leukocytes/mm 3 Platelets/mm 3 Percentage of
Prior Dose
To Be Given >4000 >100,000 100% 3000–3999 75,000–99,999 100% 2000–2999 25,000–74,999 70% <2000 <25,000 50%No repeat dosage should be given until leukocyte count has returned to 4000/mm 3 and a platelet count to 100,000/mm 3. When mitomycin is used in combination with other myelosuppressive agents, the doses should be adjusted accordingly. If the disease continues to progress after two courses of mitomycin, the drug should be stopped since chances of response are minimal.
STABILITY Unreconstituted mitomycin stored at room temperature is stable for the lot life indicated on the package. Avoid excessive heat (over 40°C, 104°F). Reconstituted with Sterile Water for Injection to a concentration of 0.5 mg per mL, mitomycin is stable for 14 days refrigerated or 7 days at room temperature. Diluted in various I.V. fluids at room temperature, to a concentration of 20 to 40 micrograms per mL: I.V. Fluid Stability 0.9% Sodium Chloride Injection 12 hours Sodium Lactate Injection 24 hours The combination of mitomycin (5 mg to 15 mg) and heparin (1,000 units to 10,000 units) in 30 mL of 0.9% Sodium Chloride Injection is stable for 48 hours at room temperature.Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published. 1-8 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
-
Accord Healthcare Inc
![Mitomycin Injection, Powder, Lyophilized, For Solution [Accord Healthcare Inc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Mitomycin | Accord Healthcare Inc
![Mitomycin Injection, Powder, Lyophilized, For Solution [Accord Healthcare Inc] Mitomycin Injection, Powder, Lyophilized, For Solution [Accord Healthcare Inc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Mitomycin should be given intravenously only, using care to avoid extravasation of the compound. If extravasation occurs, cellulitis, ulceration, and slough may result.
Each vial contains mitomycin 5 mg and mannitol 10 mg. To administer, add Sterile Water for Injection, 10 mL. Shake to dissolve. If product does not dissolve immediately, allow to stand at room temperature until solution is obtained.
After full hematological recovery (see guide to dosage adjustment) from any previous chemotherapy, the following dosage schedule may be used at 6 to 8 week intervals:
20 mg/m2 intravenously as a single dose via a functioning intravenous catheter.
Because of cumulative myelosuppression, patients should be fully reevaluated after each course of mitomycin, and the dose reduced if the patient has experienced any toxicities. Doses greater than 20 mg/m2 have not been shown to be more effective, and are more toxic than lower doses.
The following schedule is suggested as a guide to dosage adjustment:
Nadir After Prior Dose
Leukocytes/mm3
Platelets/mm3
Percentage of
Prior Dose
To Be Given>4000
>100,000
100%
3000–3999
75,000–99,999
100%
2000–2999
25,000–74,999
70%
<2000
<25,000
50%
No repeat dosage should be given until leukocyte count has returned to 4000/mm3 and a platelet count to 100,000/mm3.
When mitomycin is used in combination with other myelosuppressive agents, the doses should be adjusted accordingly. If the disease continues to progress after two courses of mitomycin, the drug should be stopped since chances of response are minimal.
STABILITY Unreconstituted mitomycin stored at room temperature is stable for the lot life indicated on the package. Avoid excessive heat (over 40°C, 104°F). Reconstituted with Sterile Water for Injection to a concentration of 0.5 mg per mL, mitomycin is stable for 14 days refrigerated or 7 days at room temperature. Dilutedin various I.V. fluids at room temperature, to a concentration of 20 to 40 micrograms per mL: I.V. Fluid Stability 0.9% Sodium Chloride Injection 12 hours Sodium Lactate Injection 24 hours The combination of mitomycin (5 mg to 15 mg) and heparin (1,000 units to 10,000 units) in 30 mL of 0.9% Sodium Chloride Injection is stable for 48 hours at room temperature.Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published.1-8 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
-
Accord Healthcare Inc
![Mitomycin Injection, Powder, Lyophilized, For Solution [Accord Healthcare Inc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Mitomycin | Accord Healthcare Inc
![Mitomycin Injection, Powder, Lyophilized, For Solution [Accord Healthcare Inc] Mitomycin Injection, Powder, Lyophilized, For Solution [Accord Healthcare Inc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Mitomycin should be given intravenously only, using care to avoid extravasation of the compound. If extravasation occurs, cellulitis, ulceration, and slough may result.
Each vial contains mitomycin 20 mg and mannitol 40 mg or mitomycin 40 mg and mannitol 80 mg. To administer, add Sterile Water for Injection, 40 mL or 80 mL respectively. Shake to dissolve. If product does not dissolve immediately, allow to stand at room temperature until solution is obtained.
After full hematological recovery (see guide to dosage adjustment) from any previous chemotherapy, the following dosage schedule may be used at 6 to 8 week intervals:
20 mg/m2 intravenously as a single dose via a functioning intravenous catheter.
Because of cumulative myelosuppression, patients should be fully reevaluated after each course of mitomycin, and the dose reduced if the patient has experienced any toxicities. Doses greater than 20 mg/m2 have not been shown to be more effective, and are more toxic than lower doses.
The following schedule is suggested as a guide to dosage adjustment:
Nadir After Prior Dose
Leukocytes/mm3
Platelets/mm3
Percentage of
Prior Dose
To Be Given>4000
>100,000
100%
3000–3999
75,000–99,999
100%
2000–2999
25,000–74,999
70%
<2000
<25,000
50%
No repeat dosage should be given until leukocyte count has returned to 4000/mm3 and a platelet count to 100,000/mm3.
When mitomycin is used in combination with other myelosuppressive agents, the doses should be adjusted accordingly. If the disease continues to progress after two courses of mitomycin, the drug should be stopped since chances of response are minimal.
STABILITY Unreconstituted mitomycin stored at room temperature is stable for the lot life indicated on the package. Avoid excessive heat (over 40°C, 104°F). Reconstituted with Sterile Water for Injection to a concentration of 0.5 mg per mL, mitomycin is stable for 14 days refrigerated or 7 days at room temperature. Dilutedin various I.V. fluids at room temperature, to a concentration of 20 to 40 micrograms per mL: I.V. Fluid Stability 0.9% Sodium Chloride Injection 12 hours Sodium Lactate Injection 24 hours The combination of mitomycin (5 mg to 15 mg) and heparin (1,000 units to 10,000 units) in 30 mL of 0.9% Sodium Chloride Injection is stable for 48 hours at room temperature.Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published.1-8 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
Login To Your Free Account


![Mitomycin Injection, Powder, Lyophilized, For Solution [Accord Healthcare Inc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=d80c6dc0-5f26-4d7a-a0f7-7698ab4f249a&name=mitomycin-carton.jpg)
![Mitomycin Injection, Powder, Lyophilized, For Solution [Accord Healthcare Inc]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=3a1300e9-ca6e-4713-b41a-bb913aed3287&name=mitomycin-carton-20mg.jpg)