Nascobal Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
Patients with early Leber's disease (hereditary optic nerve atrophy) who were treated with vitamin B12 suffered severe and swift optic atrophy.
Hypokalemia and sudden death may occur in severe megaloblastic anemia which is treated intensely with vitamin B12. Folic acid is not a substitute for vitamin B12 although it may improve vitamin B12-deficient megaloblastic anemia. Exclusive use of folic acid in treating vitamin B12-deficient megaloblastic anemia could result in progressive and irreversible neurologic damage.
Anaphylactic shock and death have been reported after parenteral vitamin B12 administration. No such reactions have been reported in clinical trials with Nascobal Nasal Spray or Nascobal Nasal Gel.
Blunted or impeded therapeutic response to vitamin B12 may be due to such conditions as infection, uremia, drugs having bone marrow suppressant properties such as chloramphenicol, and concurrent iron or folic acid deficiency.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Nascobal Nasal Spray is indicated for the maintenance of normal hematologic status in pernicious anemia patients who are in remission following intramuscular vitamin B12 therapy and who have no nervous system involvement.
Nascobal Nasal Spray is also indicated as a supplement for other vitamin B12 deficiencies, including:
I. Dietary deficiency of vitamin B12 occurring in strict vegetarians (Isolated vitamin B12 deficiency is very rare).
II. Malabsorption of vitamin B12 resulting from structural or functional damage to the stomach, where intrinsic factor is secreted, or to the ileum, where intrinsic factor facilitates vitamin B12 absorption. These conditions include HIV infection, AIDS, Crohn's disease, tropical sprue, and nontropical sprue (idiopathic steatorrhea, gluten-induced enteropathy). Folate deficiency in these patients is usually more severe than vitamin B12 deficiency.
III. Inadequate secretion of intrinsic factor, resulting from lesions that destroy the gastric mucosa (ingestion of corrosives, extensive neoplasia), and a number of conditions associated with a variable degree of gastric atrophy (such as multiple sclerosis, HIV infection, AIDS, certain endocrine disorders, iron deficiency, and subtotal gastrectomy). Total gastrectomy always produces vitamin B12 deficiency. Structural lesions leading to vitamin B12 deficiency include regional ileitis, ileal resections, malignancies, etc.
IV. Competition for vitamin B12 by intestinal parasites or bacteria. The fish tapeworm (Diphyllobothrium latum) absorbs huge quantities of vitamin B12 and infested patients often have associated gastric atrophy. The blind loop syndrome may produce deficiency of vitamin B12 or folate.
V. Inadequate utilization of vitamin B12. This may occur if antimetabolites for the vitamin are employed in the treatment of neoplasia.
It may be possible to treat the underlying disease by surgical correction of anatomic lesions leading to small bowel bacterial overgrowth, expulsion of fish tapeworm, discontinuation of drugs leading to vitamin malabsorption (see Drug/Laboratory Test Interactions), use of a gluten free diet in nontropical sprue, or administration of antibiotics in tropical sprue. Such measures remove the need for long-term administration of vitamin B12.
Requirements of vitamin B12 in excess of normal (due to pregnancy, thyrotoxicosis, hemolytic anemia, hemorrhage, malignancy, hepatic and renal disease) can usually be met with intranasal or oral supplementation.
Nascobal Nasal Spray is not suitable for vitamin B12 absorption test (Schilling Test).
History
There is currently no drug history available for this drug.
Other Information
Cyanocobalamin is a synthetic form of vitamin B12 with equivalent vitamin B12 activity. The chemical name is 5,6-dimethyl-benzimidazolyl cyanocobamide. The cobalt content is 4.35%. The molecular formula is C63H88CoN14O14P, which corresponds to a molecular weight of 1355.38 and the following structural formula:
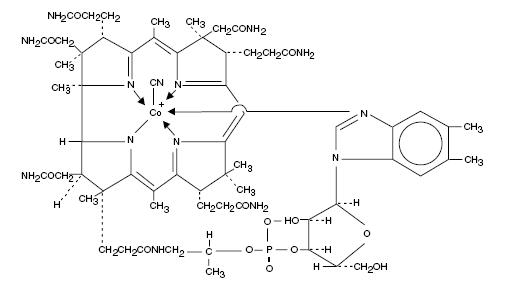
Cyanocobalamin occurs as dark red crystals or orthorhombic needles or crystalline red powder. It is very hygroscopic in the anhydrous form, and sparingly to moderately soluble in water (1:80). Its pharmacologic activity is destroyed by heavy metals (iron) and strong oxidizing or reducing agents (vitamin C), but not by autoclaving for short periods of time (15-20 minutes) at 121°C. The vitamin B12 coenzymes are very unstable in light.
Nascobal® Nasal Spray is a solution of Cyanocobalamin, USP (vitamin B12) for administration as a spray to the nasal mucosa. Each bottle of Nascobal Nasal Spray contains 2.3 mL of a 500 mcg / 0.1 mL solution of cyanocobalamin with sodium citrate, citric acid, glycerin and benzalkonium chloride in purified water. The spray solution has a pH between 4.5 and 5.5. The spray pump unit must be fully primed (see DOSAGE AND ADMINISTRATION) prior to initial use. After initial priming, each spray delivers an average of 500 mcg of cyanocobalamin and the 2.3 mL of spray solution contained in the bottle will deliver 8 doses of Nascobal Nasal Spray. The unit must be re-primed before each dose. (See DOSAGE AND ADMINISTRATION.)
Sources