Nisoldipine Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
Increased angina and/or myocardial infarction in patients with coronary artery disease: Rarely, patients, particularly those with severe obstructive coronary artery disease, have developed increased frequency, duration and/or severity of angina, or acute myocardial infarction on starting calcium channel blocker therapy or at the time of dosage increase. The mechanism of this effect has not been established. In controlled studies of Nisoldipine in patients with angina this was seen about 1.5% of the time in patients given nisoldipine, compared with 0.9% in patients given placebo.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Nisoldipine is indicated for the treatment of hypertension. It maybe used alone or in combination with other antihypertensive agents.
History
There is currently no drug history available for this drug.
Other Information
Nisoldipine is an extended release tablet dosage form of the dihydropyridine calcium channel blocker. Nisoldipine is 3,5-pyridinedicarboxylic acid, 1,4-dihydro-2,6-dimethyl-4-(2-nitrophenyl)-, methyl 2-methyl-propyl ester, C20H24N2O6, and has the structural formula:
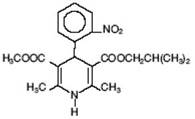
Nisoldipine is a yellow crystalline substance, practically insoluble in water but soluble in ethanol. It has a molecular weight of 388.4. Nisoldipine tablets comprise three layers: a top barrier layer, a middle layer containing nisoldipine, and a bottom barrier layer. The erodible barrier layers and the hydrogel middle layer provide for the controlled release of the drug. Nisoldipine tablets contain either 8.5, 17, 25.5, or 34 mg of nisoldipine for once-a-day oral administration.
Inactive ingredients in the formulation include: Hypromellose, hypromellose phthalate, lactose, glyceryl behenate, povidone, magnesium stearate, silicon dioxide, methacrylic acid copolymer, and sodium lauryl sulfate. Inactive ingredients in the film coating include: polydextrose, titanium dioxide, hypromellose, polyethylene glycol, iron oxide, and carnauba wax. Additionally, the 17 mg formulation contains FD&C Yellow #5.
Sources