FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Nithiodote Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
NITHIODOTE is indicated for the treatment of acute cyanide poisoning that is judged to be life-threatening. When the diagnosis of cyanide poisoning is uncertain, the potentially life-threatening risks associated with NITHIODOTE should be carefully weighed against the potential benefits, especially if the patient is not in extremis.
Cyanide poisoning may result from inhalation, ingestion, or dermal exposure to various cyanide-containing compounds, including smoke from closed-space fires. Sources of cyanide poisoning include hydrogen cyanide and its salts, cyanogenic plants, aliphatic nitriles, and prolonged exposure to sodium nitroprusside.
The presence and extent of cyanide poisoning are often initially unknown. There is no widely available, rapid, confirmatory cyanide blood test. Treatment decisions must be made on the basis of clinical history and signs and symptoms of cyanide intoxication. If clinical suspicion of cyanide poisoning is high, NITHIODOTE should be administered without delay.
| Symptoms | Signs |
|
|
In some settings, panic symptoms including tachypnea and vomiting may mimic early cyanide poisoning signs. The presence of altered mental status (e.g., confusion and disorientation) and/or mydriasis is suggestive of true cyanide poisoning although these signs can occur with other toxic exposures as well.
The expert advice of a regional poison control center may be obtained by calling 1-800-222-1222.
Smoke Inhalation
Not all smoke inhalation victims will have cyanide poisoning and may present with burns, trauma, and exposure to other toxic substances making a diagnosis of cyanide poisoning particularly difficult. Prior to administration of NITHIODOTE, smoke-inhalation victims should be assessed for the following:
- Exposure to fire or smoke in an enclosed area
- Presence of soot around the mouth, nose, or oropharynx
- Altered mental status
Although hypotension is highly suggestive of cyanide poisoning, it is only present in a small percentage of cyanide-poisoned smoke inhalation victims. Also indicative of cyanide poisoning is a plasma lactate concentration greater than or equal to 10 mmol/L (a value higher than that typically listed in the table of signs and symptoms of isolated cyanide poisoning because carbon monoxide associated with smoke inhalation also contributes to lactic acidemia). If cyanide poisoning is suspected, treatment should not be delayed to obtain a plasma lactate concentration.
Caution should be exercised when administering other cyanide antidotes simultaneously with NITHIODOTE, as the safety of co-administration has not been established. If a decision is made to administer another cyanide antidote with NITHIODOTE, these drugs should not be administered concurrently in the same IV line. [see Dosage and Administration (2.2)]
History
There is currently no drug history available for this drug.
Other Information
Sodium nitrite, one of the active ingredients in NITHIODOTE has the chemical name nitrous acid sodium salt. The chemical formula is NaNO2 and the molecular weight is 69.0. Sodium thiosulfate, the second active ingredient in NITHIODOTE has the chemical name thiosulfuric acid, disodium salt, pentahydrate. The chemical formula is Na2S2O3• 5H2O and the molecular weight is 248.17. The structural formulae are:
Structure of Sodium Nitrite

Structure of Sodium Thiosulfate Pentahydrate

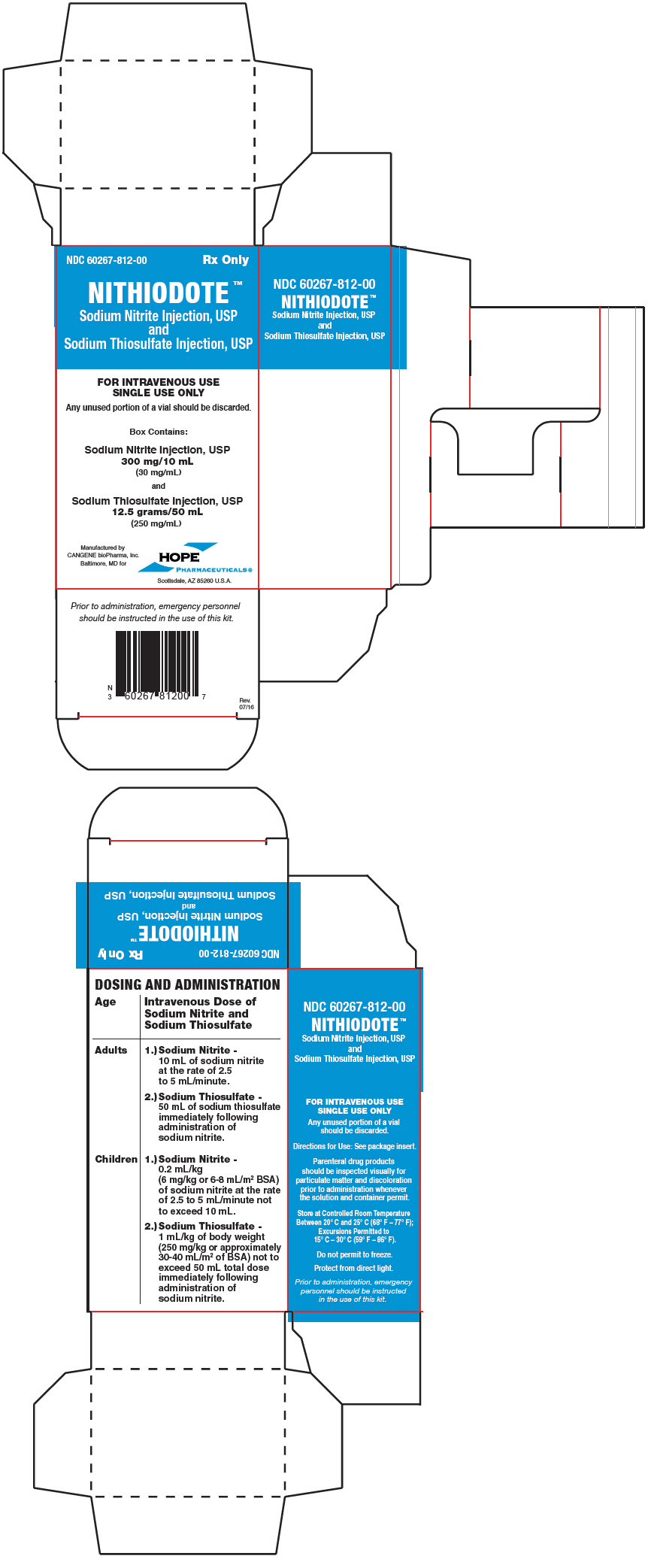
NITHIODOTE is a cyanide antidote which contains one 10 mL glass vial of a 3% solution of sodium nitrite injection and one 50 mL glass vial containing a 25% solution of sodium thiosulfate injection.
Sodium nitrite injection is a sterile aqueous solution and is intended for intravenous injection. Each vial contains 300 mg of sodium nitrite in 10 mL solution (30 mg/mL). Sodium nitrite injection is a clear solution with a pH between 7.0 and 9.0.
Sodium thiosulfate injection is a sterile aqueous solution and is intended for intravenous injection. Each vial contains 12.5 grams of sodium thiosulfate in 50 mL solution (250 mg/mL). Each mL also contains 2.8 mg boric acid and 4.4 mg of potassium chloride. The pH of the solution is adjusted with boric acid and/or sodium hydroxide. Sodium thiosulfate injection is a clear solution with a pH between 7.5 and 9.5.
Sources
Nithiodote Manufacturers
-
Hope Pharmaceuticals
![Nithiodote (Sodium Nitrite And Sodium Thiosulfate) Kit [Hope Pharmaceuticals]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Nithiodote | Hope Pharmaceuticals
![Nithiodote (Sodium Nitrite And Sodium Thiosulfate) Kit [Hope Pharmaceuticals] Nithiodote (Sodium Nitrite And Sodium Thiosulfate) Kit [Hope Pharmaceuticals]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
2.1 Administration RecommendationComprehensive treatment of acute cyanide intoxication requires support of vital functions. Administration of sodium nitrite and sodium thiosulfate should be considered adjunctive to appropriate supportive therapies. Airway, ventilatory and circulatory support, and oxygen administration should not be delayed to administer sodium nitrite and sodium thiosulfate.
Sodium nitrite injection and sodium thiosulfate injection are administered by slow intravenous injection. They should be given as early as possible after a diagnosis of acute life-threatening cyanide poisoning has been established. Sodium nitrite should be administered first, followed immediately by sodium thiosulfate. Blood pressure must be monitored during infusion in both adults and children. The rate of infusion should be decreased if significant hypotension is noted.
Age Intravenous Dose of Sodium Nitrite and Sodium Thiosulfate Adults 1.) Sodium Nitrite -10 mL of a 3% solution (300 mg) of sodium nitrite at the rate of 2.5 to 5 mL/minute 2.) Sodium Thiosulfate - 50 mL of a 25% solution (12.5 g) of a sodium thiosulfate solution immediately following administration of sodium nitrite. Children 1.) Sodium Nitrite -0.2 mL/kg of a 3% solution (6 mg/kg or 6-8 mL/m 2 BSA) of sodium nitrite at the rate of 2.5 to 5 mL/minute not to exceed 10 mL (300 mg) 2.) Sodium Thiosulfate - 1 mL/kg of body weight using a 25% solution (250 mg/kg or approximately 30-40 mL/m 2 of BSA) not to exceed 50 mL (12.5 g) total dose immediately following administration of sodium nitrite.NOTE: If signs of poisoning reappear, repeat treatment using one-half the original dose of both sodium nitrite and sodium thiosulfate.
In adult and pediatric patients with known anemia, it is recommended that the dosage of sodium nitrite should be reduced proportionately to the hemoglobin concentration. [see Warnings and Precautions (5.2)]
All parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
2.2 Recommended MonitoringPatients should be monitored for at least 24-48 hours after NITHIODOTE administration for adequacy of oxygenation and perfusion and for recurrent signs and symptoms of cyanide toxicity. When possible, hemoglobin/hematocrit should be obtained when treatment is initiated. Measurements of oxygen saturation using standard pulse oximetry and calculated oxygen saturation values based on measured PO2 are unreliable in the presence of methemoglobinemia. Methemoglobin level: Administrations of sodium nitrite solely to achieve an arbitrary level of methemoglobinemia may be unnecessary and potentially hazardous. The therapeutic effects of sodium nitrite do not appear to be mediated by methemoglobin formation alone [see Clinical Pharmacology (12)] and clinical responses to sodium nitrite administration have been reported in association with methemoglobin levels of less than 10%. Administration of sodium nitrite beyond the initial dose should be guided primarily by clinical response to treatment (i.e., a second dose should be considered only if there is inadequate clinical response to the first dose). It is generally recommended that methemoglobin concentrations be closely monitored and kept below 30%. Serum methemoglobin levels should be monitored during treatment using co-oximetry, and administration of sodium nitrite should generally be discontinued when methemoglobin levels exceed 30%. Intravenous methylene blue and exchange transfusion have been reported in the literature as treatments for life-threatening methemoglobinemia.
2.3 Incompatibility InformationChemical incompatibility has been reported between NITHIODOTE and hydroxocobalamin and these drugs should not be administered simultaneously through the same IV line. No chemical incompatibility has been reported between sodium thiosulfate and sodium nitrite, when administered sequentially through the same IV line as described in Dosage and Administration.
Login To Your Free Account