FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Nuflor Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
WARNINGS: NOT FOR HUMAN USE. KEEP OUT OF REACH OF CHILDREN. This product contains materials that can be irritating to skin and eyes. Avoid direct contact with skin, eyes, and clothing. In case of accidental eye exposure, flush with water for 15 minutes. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing. Consult a physician if irritation persists. Accidental injection of this product may cause local irritation. Consult a physician immediately. The Material Safety Data Sheet (MSDS) contains more detailed occupational safety information.
For customer service, adverse effects reporting, and/or a copy of the MSDS, call 1-800-211-3573.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
There is currently no usage information available for this product. We apologize for any inconvenience.
History
There is currently no drug history available for this drug.
Other Information
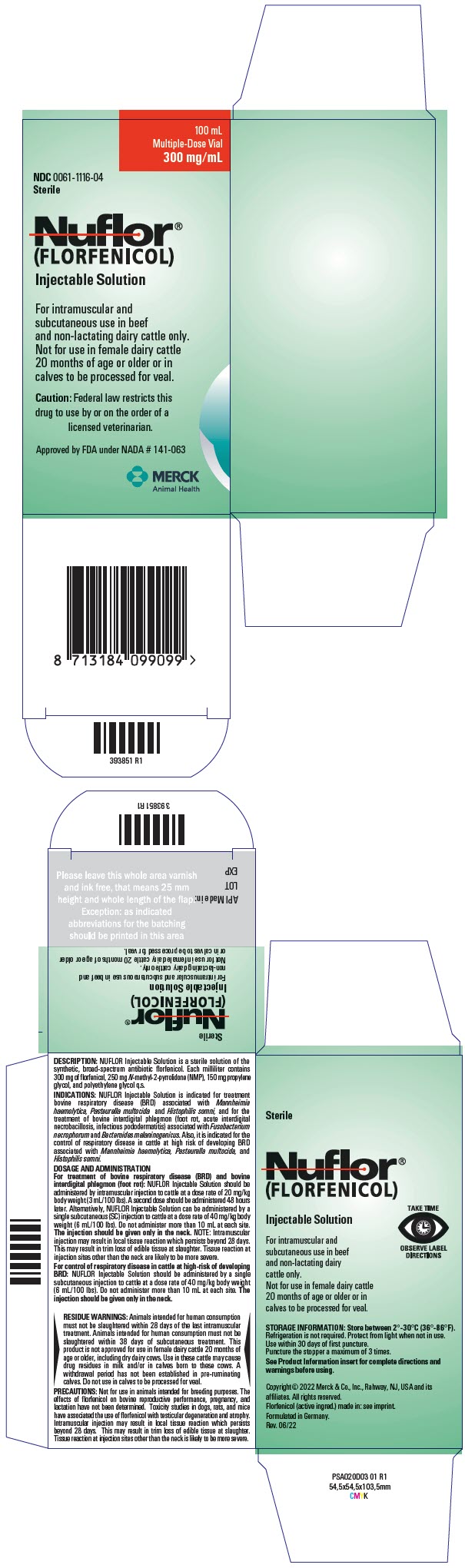
DESCRIPTION NUFLOR Injectable Solution is a solution of the synthetic antibiotic florfenicol. Each milliliter of sterile NUFLOR Injectable Solution contains 300 mg of florfenicol, 250 mg n-methyl-2-pyrrolidone, 150 mg propylene glycol, and polyethylene glycol qs.
Sources
Nuflor Manufacturers
-
Merck Sharp & Dohme Corp.
![Nuflor (Florfenicol) Injection, Solution [Merck Sharp & Dohme Corp.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Nuflor | Merck Sharp & Dohme Corp.
![Nuflor (Florfenicol) Injection, Solution [Merck Sharp & Dohme Corp.] Nuflor (Florfenicol) Injection, Solution [Merck Sharp & Dohme Corp.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
DOSAGE AND ADMINISTRATION For treatment of bovine respiratory disease (BRD) and bovine interdigital phlegmon (foot rot): NUFLOR Injectable Solution should be administered by intramuscular injection to cattle at a dose rate of 20 mg/kg body weight (3 mL/100 lbs). A second dose should be administered 48 hours later. Alternatively, NUFLOR Injectable Solution can be administered by a single subcutaneous (SC) injection to cattle at a dose rate of 40 mg/kg body weight (6 mL/100 lbs). Do not administer more than 10 mL at each site. The injection should be given only in the neck.
NOTE: Intramuscular injection may result in local tissue reaction which persists beyond 28 days. This may result in trim loss of edible tissue at slaughter. Tissue reaction at injection sites other than the neck is likely to be more severe.
For control of respiratory disease in cattle at high-risk of developing BRD: Nuflor Injectable Solution should be administered by a single subcutaneous injection to cattle at a dose rate of 40 mg/kg body weight (6 mL/100 lbs). Do not administer more than 10 mL at each site. The injection should be given only in the neck.
NUFLOR Injectable Solution DOSAGE GUIDE ANIMAL WEIGHT
(lbs) IM NUFLOR DOSAGE 3.0 mL/100 lb Body Weight
(mL) SC NUFLOR DOSAGE 6.0 mL/100 lb Body Weight
(mL) Recommended Injection Location 100 3.0 6.0 200 6.0 12.0 300 9.0 18.0 400 12.0 24.0 500 15.0 30.0 600 18.0 36.0 Do not inject more than 10 mL per injection site. 700 21.0 42.0 800 24.0 48.0 900 27.0 54.0 1000 30.0 60.0Clinical improvement should be evident in most treated subjects within 24 hours of initiation of treatment. If a positive response is not noted within 72 hours of initiation of treatment, the diagnosis should be re-evaluated.
Login To Your Free Account