FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Occuloplex I Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
PROTONIX I.V. for Injection is indicated for short-term treatment (7 to 10 days) of adult patients with gastroesophageal reflux disease (GERD) and a history of erosive esophagitis.
Safety and efficacy of PROTONIX I.V. for Injection as a treatment of patients with GERD and a history of erosive esophagitis for more than 10 days have not been demonstrated.
PROTONIX I.V. for Injection is indicated for the treatment of pathological hypersecretory conditions including Zollinger-Ellison Syndrome in adults.
History
There is currently no drug history available for this drug.
Other Information
Therapeutic class: Proton Pump Inhibitor (PPI)
Route of administration: For Intravenous use only
The active ingredient in PROTONIX® I.V. (intravenous pantoprazole sodium) for Injection is a substituted benzimidazole, sodium 5-(difluoromethoxy)-2-[[(3,4-dimethoxy-2-pyridinyl)methyl] sulfinyl]-1H-benzimidazole, a compound that inhibits gastric acid secretion. Its empirical formula is C16H14F2N3NaO4S, with a molecular weight of 405.4. The structural formula is:
Pantoprazole sodium is a white to off-white crystalline powder and is racemic. Pantoprazole has weakly basic and acidic properties. Pantoprazole sodium is freely soluble in water, very slightly soluble in phosphate buffer at pH 7.4, and practically insoluble in n-hexane. The stability of the compound in aqueous solution is pH-dependent. The rate of degradation increases with decreasing pH. The reconstituted solution of PROTONIX I.V. for Injection is in the pH range 9.0 to 10.5.
PROTONIX I.V. for Injection is supplied as a freeze-dried powder in a clear glass vial fitted with a rubber stopper and crimp seal containing pantoprazole sodium, equivalent to 40 mg of pantoprazole, edetate disodium (1 mg), and sodium hydroxide to adjust pH.
Sources
Occuloplex I Manufacturers
-
Bioactive Nutritional, Inc.
![Occuloplex I (Euphrasia Officinalis, Ginko Biloba, Hydrastis Canadensis, Taraxacum Officinale, Beta Carotene, Cyanocobalamin, Glutathione) Liquid [Bioactive Nutritional, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Occuloplex I | Chattem, Inc.
![Occuloplex I (Euphrasia Officinalis, Ginko Biloba, Hydrastis Canadensis, Taraxacum Officinale, Beta Carotene, Cyanocobalamin, Glutathione) Liquid [Bioactive Nutritional, Inc.] Occuloplex I (Euphrasia Officinalis, Ginko Biloba, Hydrastis Canadensis, Taraxacum Officinale, Beta Carotene, Cyanocobalamin, Glutathione) Liquid [Bioactive Nutritional, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
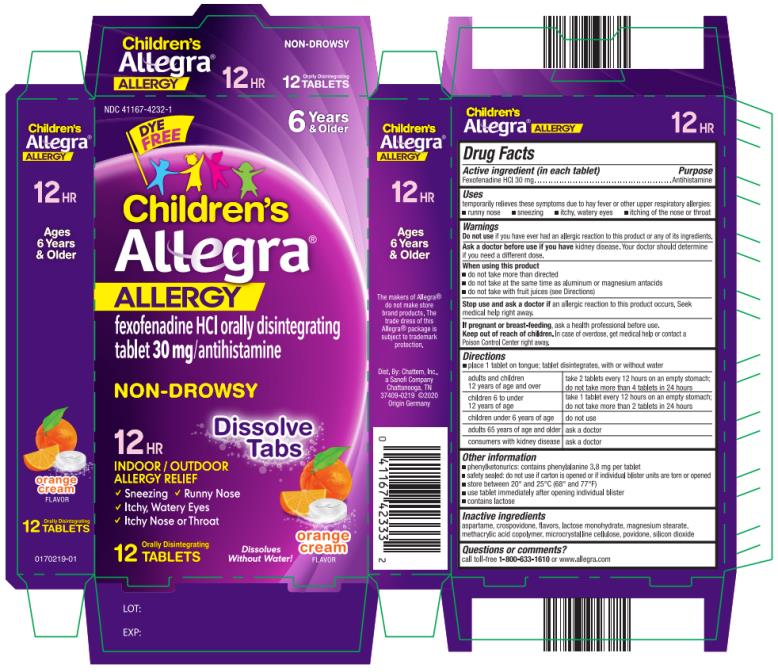
place 1 tablet on tongue; tablet disintegrates, with or without water adults and children
12 years of age and over take 2 tablets every 12 hours on an empty stomach;
do not take more than 4 tablets in 24 hours children 6 to under
12 years of age take 1 tablet every 12 hours on an empty stomach;
do not take more than 2 tablets in 24 hours children under 6 years of age do not use adults 65 years of age and older ask a doctor consumers with kidney disease ask a doctor
Login To Your Free Account