FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Osphos Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
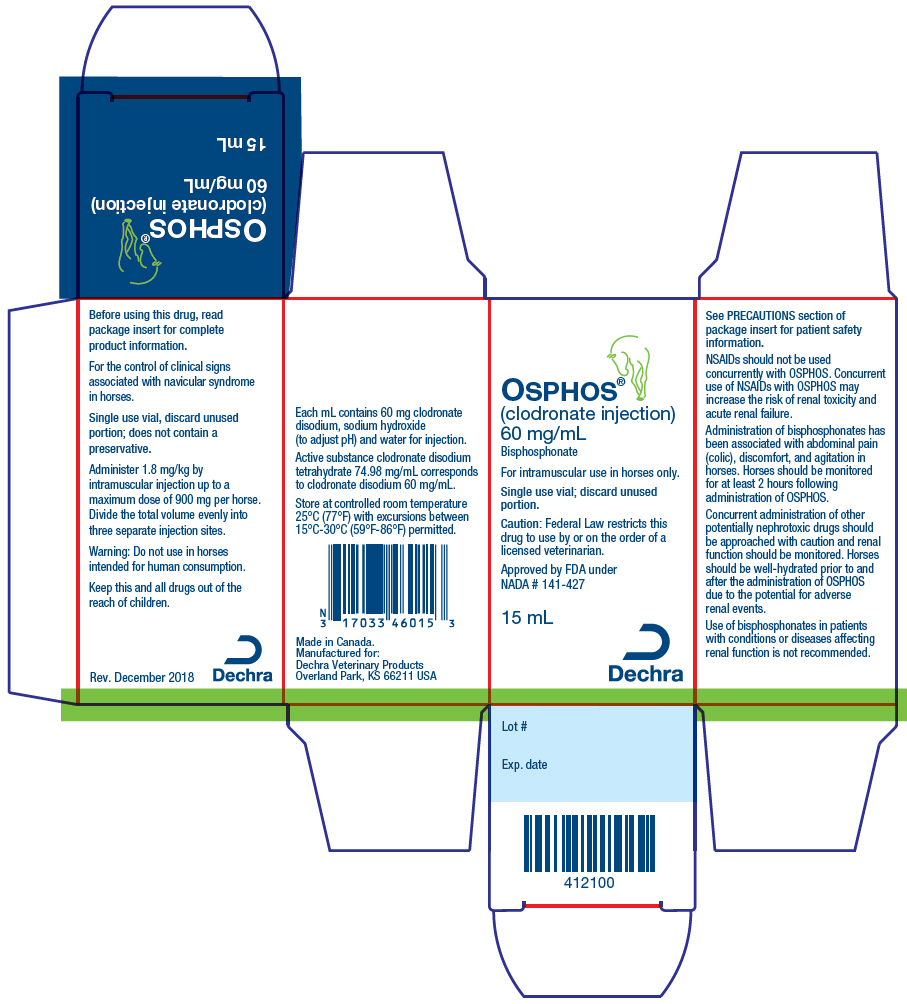
Federal law (USA) restricts this drug to use by or on the order of a licensed veterinarian.
For intramuscular use in horses only.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
For the control of clinical signs associated with navicular syndrome in horses.
History
There is currently no drug history available for this drug.
Other Information
Clodronate disodium is a non-amino, chloro-containing bisphosphonate. Chemically, clodronate disodium is (dichloromethylene) diphosphonic acid disodium salt and is manufactured from the tetrahydrate form.
The structural formula of clodronate disodium is:

Molecular Formula: CH2CL2O6 P2.2Na Molecular Weight: 288.85
Active substance clodronate disodium tetrahydrate 74.98 mg/mL corresponds to clodronate disodium 60.0 mg/mL. Each mL contains 60 mg clodronate disodium, sodium hydroxide (to adjust pH) and water for injection.
Sources
Osphos Manufacturers
-
Dechra Veterinary Products
![Osphos (Clodronate Disodium) Injection, Solution [Dechra Veterinary Products]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Osphos | Dechra Veterinary Products
![Osphos (Clodronate Disodium) Injection, Solution [Dechra Veterinary Products] Osphos (Clodronate Disodium) Injection, Solution [Dechra Veterinary Products]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Administer 1.8 mg/kg by intramuscular injection up to a maximum dose of 900 mg per horse. Divide the total volume evenly into three separate injection sites. Discard unused vial contents. OSPHOS is provided in a single use vial and does not contain a preservative.
Clinical improvement is most evident at 2 months post-treatment (see Effectiveness). Of the horses that responded to treatment with OSPHOS in the field study, 65% maintained their level of improvement through the 6 month evaluation.
If there is no response to initial therapy, the horse should be re-evaluated. For horses that initially respond to OSPHOS but do not maintain their clinical improvement for 6 months, OSPHOS may be re-administered at 3 to 6 month intervals based on recurrence of clinical signs. For horses that respond to OSPHOS and maintain clinical improvement for 6 months, OSPHOS should be re-administered after clinical signs recur.
Login To Your Free Account