Pataday Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
For topical ocular use only. Not for injection or oral use.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
PATADAY™solution is indicated for the treatment of ocular itching associated with allergic conjunctivitis.
History
There is currently no drug history available for this drug.
Other Information
PATADAY™ (olopatadine hydrochloride ophthalmic solution) 0.2% is a sterile ophthalmic solution containing olopatadine for topical administration to the eyes.
Olopatadine hydrochloride is a white, crystalline, water-soluble powder with a molecular weight of 373.88 and a molecular formula of C21H23NO3· HCl. The chemical structure is presented below:
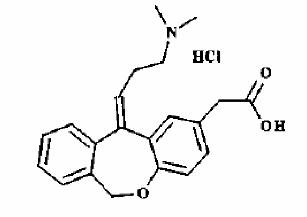
Chemical Name: 11-[(Z)-3-(Dimethylamino) propylidene]-6-11-dihydrodibenz[b,e] oxepin-2-acetic acid, hydrochloride
Each mL of PATADAY™ solution contains: Active: 2.22 mg olopatadine hydrochloride equivalent to 2 mg olopatadine. Inactives: povidone; dibasic sodium phosphate; sodium chloride; edetate disodium; benzalkonium chloride 0.01% (preservative) hydrochloric acid / sodium hydroxide (adjust pH); and purified water.
It has a pH of approximately 7 and an osmolality of approximately 300 mOsm/kg.
Sources