FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Pentazocine Hydrochloride And Acetaminophen Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
Hepatotoxicity
Pentazocine hydrochloride and acetaminophen tablets contain acetaminophen and pentazocine hydrochloride. Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4000 milligrams per day, often involve more than one acetaminophen-containing product. The excessive intake of acetaminophen may be intentional to cause self-harm or unintentional as patients attempt to obtain more pain relief or unknowingly take other acetaminophen-containing products.
The risk of acute liver failure is higher in individuals with underlying liver disease and in individuals who ingest alcohol while taking acetaminophen.
Instruct patients to look for acetaminophen or APAP on package labels and not to use more than one product that contains acetaminophen. Instruct patients to seek medical attention immediately upon ingestion of more than 4000 milligrams of acetaminophen per day, even if they feel well.
Hypersensitivity/anaphylaxis
There have been post-marketing reports of hypersensitivity and anaphylaxis associated with use of acetaminophen. Clinical signs included swelling of the face, mouth, and throat, respiratory distress, urticaria, rash, pruritus, and vomiting. There were infrequent reports of life-threatening anaphylaxis requiring emergent medical attention. Instruct patients to discontinue pentazocine hydrochloride and acetaminophen tablets immediately and seek medical care if they experience these symptoms. Do not prescribe pentazocine hydrochloride and acetaminophen tablets for patients with acetaminophen allergy.
Drug Dependence
Pentazocine can cause a physical and psychological dependence. (See DRUG ABUSE AND DEPENDENCE.)
Use In Head Injury and Increased Intracranial Pressure
In the presence of head injury, intracranial lesions or a preexisting increase in intracranial pressure, the possible respiratory depressant effects of pentazocine and its potential to elevate cerebrospinal fluid pressure (resulting from vasodilation following CO2 retention) may be markedly increased. Furthermore, pentazocine can produce effects on pupillary response and consciousness, which may obscure neurologic signs of further increases in intracranial pressure in patients with head injuries. In such patients, pentazocine hydrochloride and acetaminophen tablets must be used with extreme caution and only if its use is deemed essential.
Interactions with Alcohol and Drugs of Abuse
Pentazocine may be expected to have additive effects when used in conjunction with alcohol, other opioids, or illicit drugs that cause central nervous system depression because respiratory depression, hypotension, profound sedation, coma or death may result.
Patients Receiving Narcotics
Pentazocine is a mild narcotic antagonist. Some patients previously given narcotics, including methadone for the daily treatment of narcotic dependence, have experienced withdrawal symptoms after receiving pentazocine.
Respiratory Depression
Respiratory depression occurs more frequently in elderly or debilitated patients and in those suffering from conditions accompanied by hypoxia, hypercapnia, or upper airway obstruction, in whom even moderate therapeutic doses may significantly decrease pulmonary ventilation. Use pentazocine hydrochloride and acetaminophen tablets with extreme caution in patients with chronic obstructive pulmonary disease or cor pulmonale and in patients having a substantially decreased respiratory reserve (e.g., severe kyphoscoliosis), hypoxia, hypercapnia, or pre-existing respiratory depression. Alternative non-opioid analgesics should be considered, and pentazocine hydrochloride and acetaminophen tablets should be employed only under careful medical supervision at the lowest effective dose in such patients.
Acute CNS Manifestations
Patients receiving therapeutic doses of pentazocine hydrochloride and acetaminophen tablets have experienced hallucinations (usually visual), disorientation, and confusion which have cleared spontaneously within a period of hours. The mechanism of this reaction is not known. Such patients should be closely observed and vital signs checked. If the drug is reinstituted, it should be done with caution since these acute CNS manifestations may recur.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Pentazocine Hydrochloride and Acetaminophen Tablets are indicated for the relief of mild to moderate pain.
History
There is currently no drug history available for this drug.
Other Information
Pentazocine Hydrochloride and Acetaminophen Tablets are a combination of pentazocine hydrochloride, USP, equivalent to 25 mg base and acetaminophen, USP, 650 mg.
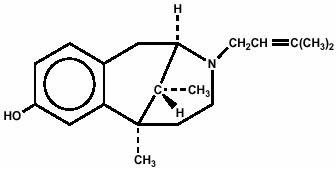
Pentazocine is a member of the benzazocine series (also known as the benzomorphan series). Chemically, pentazocine is (2R*,6R*,11R*)1,2,3,4,5,6-hexahydro-6,11-dimethyl-3-(3-methyl-2-butenyl)-2,6-methano-3-benzazocin-8-ol, a white, crystalline substance soluble in acidic aqueous solutions, and has the following structural formula:

C19H27NO HCl M.W. 321.88
Chemically, acetaminophen is Acetamide, N-(4-hydroxyphenyl)-, and has the following structural formula:

C8H9NO2 M.W. 151.16
Pentazocine is an analgesic and acetaminophen is an analgesic and antipyretic.
Inactive ingredients: colloidal silicon dioxide, FD&C Blue # 1 aluminum lake, microcrystalline cellulose, sodium lauryl sulfate, sodium starch glycolate, and stearic acid.
Sources
Pentazocine Hydrochloride And Acetaminophen Manufacturers
-
Gavis Pharmaceuticals, Llc
![Pentazocine Hydrochloride And Acetaminophen Tablet [Gavis Pharmaceuticals, Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Pentazocine Hydrochloride And Acetaminophen | Gavis Pharmaceuticals, Llc
![Pentazocine Hydrochloride And Acetaminophen Tablet [Gavis Pharmaceuticals, Llc] Pentazocine Hydrochloride And Acetaminophen Tablet [Gavis Pharmaceuticals, Llc]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Adult
The usual adult dose is 1 caplet every 4 hours as needed for pain relief, up to a maximum of 6 caplets per day.
Discontinuation
Due to the potential for withdrawal symptoms associated with abrupt discontinuation, consideration should be given to tapering patients off pentazocine hydrochloride and acetaminophen tablets after prolonged periods of treatment with pentazocine hydrochloride and acetaminophen tablets (See PRECAUTIONS, Drug Abuse and Dependence).
Login To Your Free Account