Riluzole Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
Liver Injury / Monitoring Liver Chemistries
Riluzole tablets should be prescribed with care in patients with current evidence or history of abnormal liver function indicated by significant abnormalities in serum transaminase (ALT/SGPT; AST/SGOT), bilirubin, and/or gamma-glutamate transferase (GGT) levels (see PRECAUTIONS and DOSAGE AND ADMINISTRATION sections). Baseline elevations of several LFTs (especially elevated bilirubin) should preclude the use of riluzole tablets.
Riluzole tablets even in patients without a prior history of liver disease causes serum aminotransferase elevations. Treatment should be discontinued if ALT levels are ≥ 5 X ULN or if clinical jaundice develops.
Experience in almost 800 ALS patients indicates that about 50% of riluzole-trated patients will experience at least one ALT/SGPT level above the upper limit of normal, about 8% will have elevation > 3 X ULN, and about 2% of patients will have elevations >5 X ULN. A single non-ALS patient with epilepsy treated with concomitant carbamazepine and phenobarbital experienced marked, rapid elevation of liver enzymes with jaundice (ALT 26 X ULN, AST 17 X ULN, and bilirubin 11 X ULN) four months after starting riluzole tablets; these returned to normal 7 weeks after treatment discontinuation.
Maximum increases in serum ALT usually occurred within 3 months after the start of riluzole therapy and were usually transient when < 5 times ULN. In trials, if ALT levels were < 5 times ULN, treatment continued and ALT levels usually returned to below 2 times ULN within 2 to 6 months. Treatment in studies was discontinued, however, if ALT levels exceeded 5 X ULN, so that there is no experience with continued treatment of ALS patients once ALT values exceed 5 times ULN. There were rare instances of jaundice. There is limited experience with rechallenge of patients who have had riluzole tablets discontinued for ALT > 5 X ULN, but there is the possibility of increased ALT values reoccurring (see PRECAUTIONS: Laboratory Tests). Therefore, rechallenge is not recommended.
In postmarketing experience, cases of clinical hepatitis associated with riluzole have been reported, including with fatal outcome.
Neutropenia
Among approximately 4000 patients given riluzole for ALS, there were three cases of marked neutropenia (absolute neutrophil count less than 500/mm3), all seen within the first 2 months of riluzole treatment. In one case, neutrophil counts rose on continued treatment. In a second case, counts rose after therapy was stopped. A third case was more complex, with marked anemia as well as neutropenia and the etiology of both is uncertain. Patients should be warned to report any febrile illness to their physicians. The report of a febrile illness should prompt treating physicians to check white blood cell counts.
Interstitial Lung Disease
Cases of interstitial lung disease (see ADVERSE REACTIONS) have been reported in patients treated with riluzole, some of them severe; upon further investigation, many of these cases were hypersensitivity pneumonitis. If respiratory symptoms develop such as dry cough and/or dyspnea, chest radiography should be performed, and in case of findings suggestive of interstitial lung disease or hypersensitivity pneumonitis (e.g., bilateral diffuse lung opacities), riluzole should be discontinued immediately. In the majority of the reported cases, symptoms resolved after drug discontinuation and symptomatic treatment.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
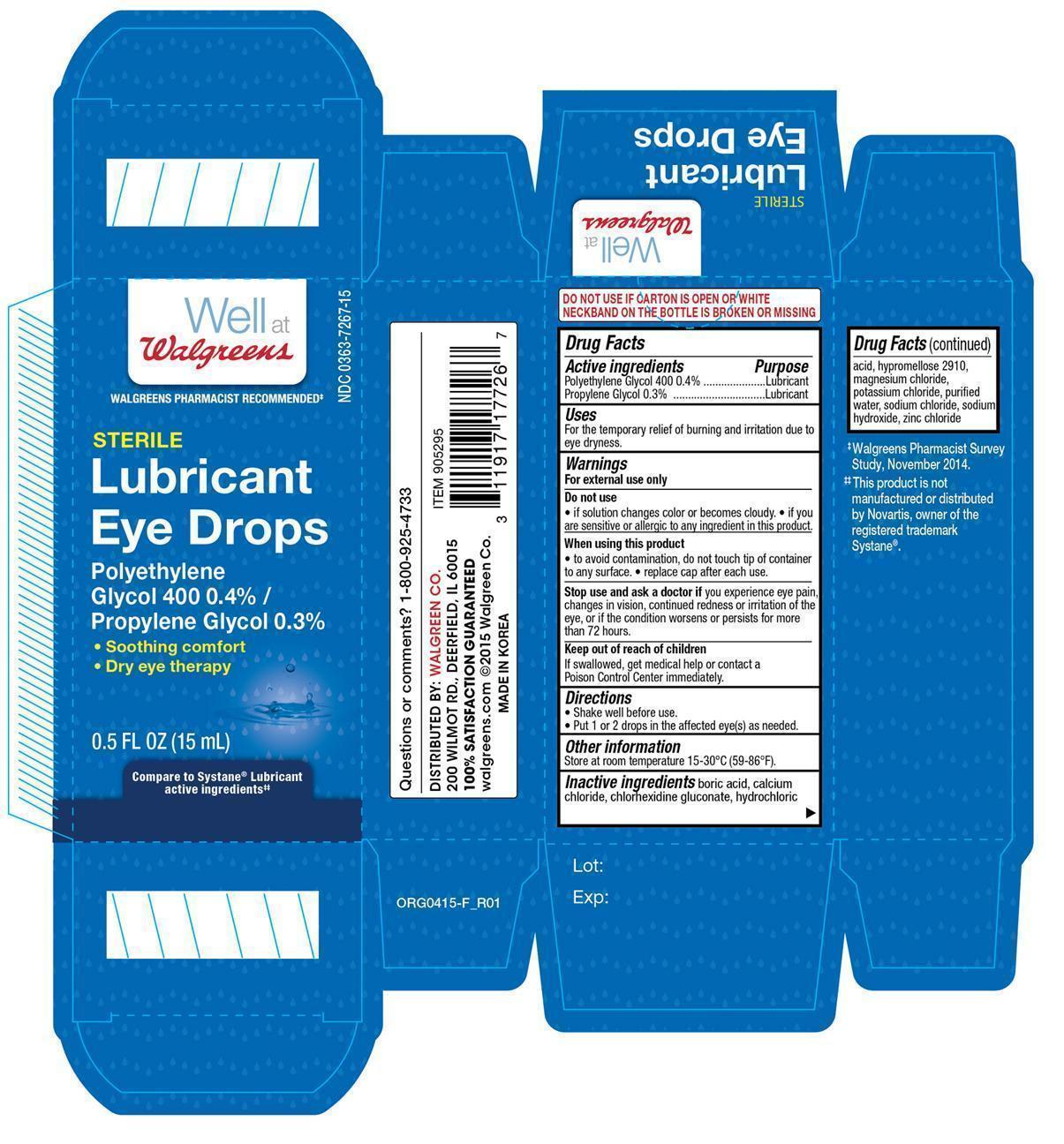
Uses
Riluzole tablets, USP are indicated for the treatment of patients with amyotrophic lateral sclerosis (ALS). Riluzole extends survival and/or time to tracheostomy.
History
There is currently no drug history available for this drug.
Other Information
Riluzole is a member of the benzothiazole class. Chemically, riluzole is 2-amino-6-(trifluoromethoxy)benzothiazole. Its molecular formula is C8H5F3N2OS and its molecular weight is 234.2. Its structural formula is as follows:

Riluzole, USP is a white to slightly yellow powder that is very soluble in dimethylformamide, dimethylsulfoxide and methanol, freely soluble in dichloromethane, sparingly soluble in 0.1 N HCl and very slightly soluble in water and in 0.1 N NaOH. Riluzole tablets, USP is available as a capsule-shaped, white to off-white, film-coated, biconvex tablet for oral administration containing 50 mg of riluzole. Each tablet is debossed with “APO” on one side and “RI-50” on the other side.
Inactive Ingredients
Core: dibasic calcium phosphate dihydrate, microcrystalline cellulose, magnesium stearate, croscarmellose sodium.
Film coating: hypromellose, polyethylene glycol, talc, titanium dioxide.
Sources


![Riluzole Tablet, Film Coated [Rising Pharmaceuticals, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=f3c2360d-cec4-4a4a-83b6-35e0114d2bc3&name=a73367a9-124a-45a9-b93b-c8ee4f87a8ab-04.jpg)
![Riluzole Tablet, Film Coated [Sun Pharmaceutical Industries Limited ]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=4c851be4-f0d0-4b24-baf9-427ddfc6f7b0&name=label.jpg)
![Riluzole Tablet, Film Coated [Apotex Corp]](http://recallguide.cwdevelopsp.com/wp-content/themes/recallguide/assets/img/drug-image-placeholder.jpg)
![Riluzole Tablet, Film Coated [Glenmark Generics Inc.,usa]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=f1ce57e8-d3fe-422c-8724-3101c258898a&name=f1ce57e8-d3fe-422c-8724-3101c258898a-05.jpg)
![Riluzole Tablet, Film Coated [Mylan Pharmaceuticals Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=97457622-5727-4b07-9e72-edd784b2d76d&name=5ce58f97-db12-4114-b006-ae60ff35ebcc-04.jpg)
![Riluzole Tablet, Film Coated [Global Pharmaceuticals, Division Of Impax Laboratories, Inc.]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=ace12152-117c-455b-8e68-e6325657569e&name=riluzole-04.jpg)
![Riluzole Tablet, Film Coated [Kaiser Foundation Hospitals]](http://dailymed.nlm.nih.gov/dailymed/image.cfm?setid=1e751a55-f807-48c7-ab4b-ec8d42770bb9&name=riluzole-04.jpg)