FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Rotarix Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
ROTARIX® is indicated for the prevention of rotavirus gastroenteritis caused by G1 and non-G1 types (G3, G4, and G9) when administered as a 2-dose series [see Clinical Studies (14.3)]. ROTARIX is approved for use in infants 6 weeks to 24 weeks of age.
History
There is currently no drug history available for this drug.
Other Information
ROTARIX (Rotavirus Vaccine, Live, Oral), for oral administration, is a live, attenuated rotavirus vaccine derived from the human 89-12 strain which belongs to G1P[8] type. The rotavirus strain is propagated on Vero cells. After reconstitution, the final formulation (1 mL) contains at least 106.0 median Cell Culture Infective Dose (CCID50) of live, attenuated rotavirus.
The lyophilized vaccine contains amino acids, dextran, Dulbecco’s Modified Eagle Medium (DMEM), sorbitol, and sucrose. DMEM contains the following ingredients: sodium chloride, potassium chloride, magnesium sulfate, ferric (III) nitrate, sodium phosphate, sodium pyruvate, D-glucose, concentrated vitamin solution, L-cystine, L-tyrosine, amino acids solution, L-glutamine, calcium chloride, sodium hydrogenocarbonate, and phenol red.
In the manufacturing process, porcine-derived materials are used. Porcine circovirus type 1 (PCV-1) is present in ROTARIX. PCV-1 is not known to cause disease in humans.
The liquid diluent contains calcium carbonate, sterile water, and xanthan. The diluent includes an antacid component (calcium carbonate) to protect the vaccine during passage through the stomach and prevent its inactivation due to the acidic environment of the stomach.
ROTARIX is available in single-dose vials of lyophilized vaccine, accompanied by a prefilled oral applicator of liquid diluent [see How Supplied/Storage and Handling (16)]. The tip caps of the prefilled oral applicators may contain natural rubber latex; the vial stoppers are not made with natural rubber latex.
ROTARIX contains no preservatives.
Sources
Rotarix Manufacturers
-
Glaxosmithkline Biologicals Sa
![Rotarix (Rotavirus Vaccine, Live, Oral) Kit [Glaxosmithkline Biologicals Sa]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Rotarix | Glaxosmithkline Biologicals Sa
![Rotarix (Rotavirus Vaccine, Live, Oral) Kit [Glaxosmithkline Biologicals Sa] Rotarix (Rotavirus Vaccine, Live, Oral) Kit [Glaxosmithkline Biologicals Sa]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
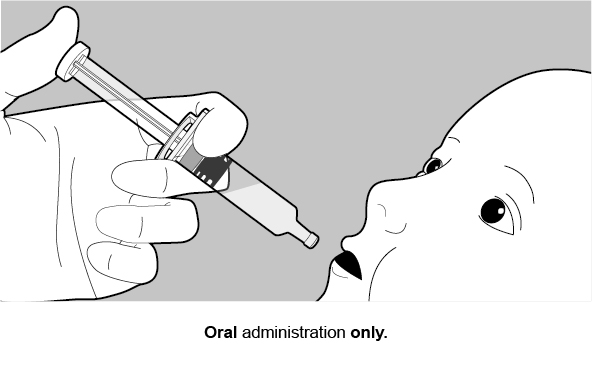
2.1 Reconstitution Instructions for Oral AdministrationFor oral use only. Not for injection.
Reconstitute only with accompanying diluent. Do not mix ROTARIX with other vaccines or solutions.
Remove vial cap and push transfer adapter onto vial (lyophilized vaccine).
Shake diluent in oral applicator (white, turbid suspension). Connect oral applicator to transfer adapter.
Push plunger of oral applicator to transfer diluent into vial. Suspension will appear white and turbid.
Withdraw vaccine into oral applicator.
Twist and remove the oral applicator.
Ready for oral administration.
Do not use a needle with ROTARIX.
Not for injection.
2.2 Recommended Dose and ScheduleThe vaccination series consists of two 1-mL doses administered orally. The first dose should be administered to infants beginning at 6 weeks of age. There should be an interval of at least 4 weeks between the first and second dose. The 2-dose series should be completed by 24 weeks of age.
Safety and effectiveness have not been evaluated if ROTARIX were administered for the first dose and another rotavirus vaccine were administered for the second dose or vice versa.
In the event that the infant spits out or regurgitates most of the vaccine dose, a single replacement dose may be considered at the same vaccination visit.
2.3 Infant FeedingBreast-feeding was permitted in clinical studies. There was no evidence to suggest that breast-feeding reduced the protection against rotavirus gastroenteritis afforded by ROTARIX. There are no restrictions on the infant’s liquid consumption, including breast-milk, either before or after vaccination with ROTARIX.
Login To Your Free Account