FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Saizen Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Saizen® [somatropin (rDNA origin) for injection] is indicated for the treatment of pediatric patients with growth failure due to inadequate secretion of endogenous growth hormone.
Saizen® is indicated for replacement of endogenous growth hormone in adults with growth hormone deficiency who meet either of the following two criteria:
Adult Onset
Patients who have growth hormone deficiency, either alone or associated with multiple hormone deficiencies (hypopituitarism), as a result of pituitary disease, hypothalamic disease, surgery, radiation therapy, or trauma; or
Childhood Onset
Patients who were growth hormone deficient during childhood as a result of congenital, genetic, acquired, or idiopathic causes.
Patients who were treated with somatropin for growth hormone deficiency in childhood and whose epiphyses are closed should be reevaluated before continuation of somatropin therapy at the reduced dose level recommended for growth hormone deficient adults. Confirmation of the diagnosis of adult growth hormone deficiency in both groups involves an appropriate growth hormone provocative test with two exceptions: (1) patients with multiple other pituitary hormone deficiencies due to organic disease; and (2) patients with congenital/genetic growth hormone deficiency.
History
There is currently no drug history available for this drug.
Other Information
Saizen® is a human growth hormone produced by recombinant DNA technology. Saizen® has 191 amino acid residues and a molecular weight of 22,125 daltons. Its amino acid sequence and structure are identical to the dominant form of human pituitary growth hormone. Saizen® is produced by a mammalian cell line (mouse C127) that has been modified by the addition of the human growth hormone gene.
Saizen® is a sterile, non pyrogenic, white, lyophilized powder intended for subcutaneous injection after reconstitution with Bacteriostatic Water for Injection, USP (0.9% Benzyl Alcohol). The reconstituted solution has a pH of 6.5 to 8.5.
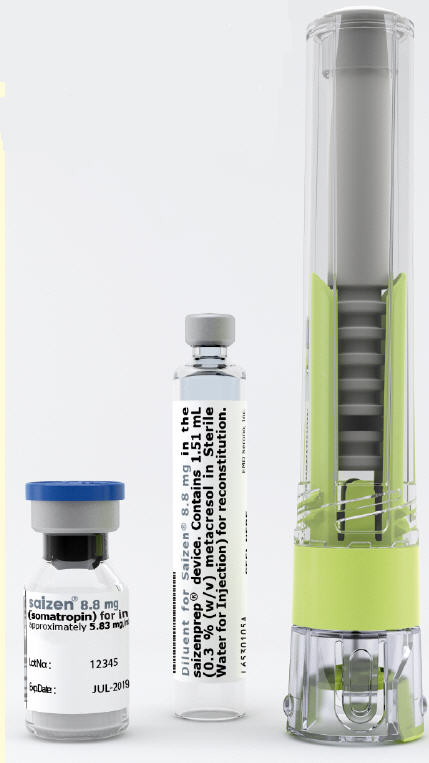
Vials
Saizen® is available in 5 mg and 8.8 mg vials. The quantitative composition per vial is:
5 mg vial:
Each vial contains 5.0 mg somatropin, 34.2 mg sucrose and 1.16 mg O-phosphoric acid. The pH is adjusted with sodium hydroxide or O-phosphoric acid.
8.8 mg vial:
Each vial contains 8.8 mg somatropin, 60.2 mg sucrose and 2.05 mg O-phosphoric acid. The pH is adjusted with sodium hydroxide or O-phosphoric acid.
Diluent
The diluent is Bacteriostatic Water for Injection, USP containing 0.9% Benzyl Alcohol added as an antimicrobial preservative.
click.easy® reconstitution device
Saizen® is also available in the click.easy® reconstitution device. The quantitative composition per vial contained in the click.easy® reconstitution device is:
8.8 mg vial contained in the click.easy® device
Each vial contains 8.8 mg somatropin, 60.2 mg sucrose and 2.05 mg O-phosphoric acid. The pH is adjusted with sodium hydroxide or O-phosphoric acid.
Diluent
The diluent contained in click.easy® device is 0.3% (w/v) metacresol in Sterile Water for Injection added as an antimicrobial preservative. The reconstituted solution has a pH of 6.5 to 8.5.
Sources
Saizen Manufacturers
-
Emd Serono, Inc.
![Saizen (Somatropin) Kit Saizen Clickeasy (Somatropin) Kit [Emd Serono, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Saizen | Emd Serono, Inc.
![Saizen (Somatropin) Kit Saizen Clickeasy (Somatropin) Kit [Emd Serono, Inc.] Saizen (Somatropin) Kit Saizen Clickeasy (Somatropin) Kit [Emd Serono, Inc.]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
For subcutaneous injection.
Saizen® therapy should be supervised by a physician who is experienced in the diagnosis and management of pediatric patients with growth hormone deficiency or adult patients with either childhood-onset or adult-onset growth hormone deficiency.
2.1 Pediatric Growth Hormone Deficiency (GHD)Saizen® dosage and administration schedule should be individualized for each patient. The recommended weekly dosage is 0.18 mg/kg of body weight by subcutaneous injection. It should be divided into equal doses given either on 3 alternate days, 6 times per week or daily.
Response to somatropin therapy in pediatric patients tends to decrease with time. However, in pediatric patients, the failure to increase growth rate, particularly during the first year of therapy, indicates the need for close assessment of compliance and evaluation for other causes of growth failure, such as hypothyroidism, undernutrition, advanced bone age and antibodies to recombinant human growth hormone.
Treatment with Saizen® of growth failure due to growth hormone deficiency should be discontinued when the epiphyses are fused.
2.2 Adult Growth Hormone Deficiency (GHD)Either of two approaches to Saizen® dosing may be followed: a weight-based regimen or a non-weight-based regimen.
Weight-based
Based on the dosing utilized in the original pivotal study described herein, the recommended dosage at the start of therapy is not more than 0.005 mg/kg given as a daily subcutaneous injection. The dosage may be increased to not more than 0.01 mg/kg/day after 4 weeks according to individual patient requirements. Clinical response, side effects, and determination of age-and gender-adjusted serum insulin-like growth factor (IGF-1) levels may be used as guidance in dose titration.
Non-weight-based
Alternatively, taking into account more recent literature, a starting dose of approximately 0.2 mg/day (range, 0.15-0.30 mg/day) may be used without consideration of body weight. This dose can be increased gradually every 1 to 2 months by increments of approximately 0.1 to 0.2 mg/day, according to individual patient requirements based on the clinical response and serum IGF-1 concentrations. During therapy, the dose should be decreased if required by the occurrence of adverse reactions and/or serum IGF-1 levels above the age- and gender-specific normal range. Maintenance dosages vary considerably from person to person.
A lower starting dose and smaller dose increments should be considered for older patients, who are more prone to the adverse effects of somatropin than younger individuals. In addition, obese individuals are more likely to manifest adverse effects when treated with a weight-based regimen. In order to reach the defined treatment goal, estrogen-replete women may need higher doses than men. Oral estrogen administration may increase the dose requirements in women.
2.3 Preparation and AdministrationVials
To prevent possible contamination, wipe the rubber vial stopper with an antiseptic solution before puncturing it with the needle. It is recommended that Saizen® be administered using sterile, disposable syringes and needles. The syringes should be of small enough volume that the prescribed dose can be drawn from the vial with reasonable accuracy.
After determining the appropriate patient dose, reconstitute each vial of Saizen® as follows: 5 mg vial with 1 to 3 mL of Bacteriostatic Water for Injection, USP (Benzyl Alcohol preserved); 8.8 mg vial with 2-3 mL of Bacteriostatic Water for Injection, USP (Benzyl Alcohol preserved). Approximately 10% mechanical loss can be associated with reconstitution and multidose administration.
If sensitivity to the diluent occurs, Saizen® may be reconstituted with Sterile Water for Injection, USP. When Saizen® is reconstituted in this manner, the reconstituted solution should be used immediately and any unused solution should be discarded [see Warnings and Precautions (5.15)].
To reconstitute Saizen®, inject the diluent into the vial of Saizen® aiming the liquid against the glass vial wall. Swirl the vial with a GENTLE rotary motion until contents are dissolved completely. DO NOT SHAKE. Parenteral drug products should always be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. SAIZEN MUST NOT BE INJECTED if the solution is cloudy or contains particulate matter. Use it only if it is clear and colorless.
click.easy® cartridges
For drug preparation instructions for Saizen® click.easy® cartridges, please refer to the instructions for use provided with click.easy® reconstitution device.
Login To Your Free Account