FDA records indicate that there are no current recalls for this drug.
Are you a medical professional?
Trending Topics
Sandostatin Lar Depot Recall
Get an alert when a recall is issued.
Questions & Answers
Side Effects & Adverse Reactions
There is currently no warning information available for this product. We apologize for any inconvenience.
Legal Issues
There is currently no legal information available for this drug.
FDA Safety Alerts
There are currently no FDA safety alerts available for this drug.
Manufacturer Warnings
There is currently no manufacturer warning information available for this drug.
FDA Labeling Changes
There are currently no FDA labeling changes available for this drug.
Uses
Sandostatin LAR Depot 10 mg, 20 mg, and 30 mg is indicated in patients in whom initial treatment with Sandostatin Injection has been shown to be effective and tolerated.
Long-term maintenance therapy in acromegalic patients who have had an inadequate response to surgery and/or radiotherapy, or for whom surgery and/or radiotherapy is not an option. The goal of treatment in acromegaly is to reduce GH and IGF-1 levels to normal [see Clinical Studies (14) and Dosage and Administration (2)].
Long-term treatment of the severe diarrhea and flushing episodes associated with metastatic carcinoid tumors.
Long-term treatment of the profuse watery diarrhea associated with VIP-secreting tumors.
In patients with carcinoid syndrome and VIPomas, the effect of Sandostatin Injection and Sandostatin LAR Depot on tumor size, rate of growth and development of metastases, has not been determined.
History
There is currently no drug history available for this drug.
Other Information
Octreotide is the acetate salt of a cyclic octapeptide. It is a long-acting octapeptide with pharmacologic properties mimicking those of the natural hormone somatostatin. Octreotide is known chemically as L-Cysteinamide, D-phenylalanyl-L-cysteinyl-L-phenylalanyl-D-tryptophyl-L-lysyl-L-threonyl-N-[2-hydroxy-1- (hydroxy-methyl) propyl]-, cyclic (2→7)-disulfide; [R-(R*,R*)].
The molecular weight of octreotide is 1019.3 (free peptide, C49H66N10O10S2) and its amino acid sequence is:

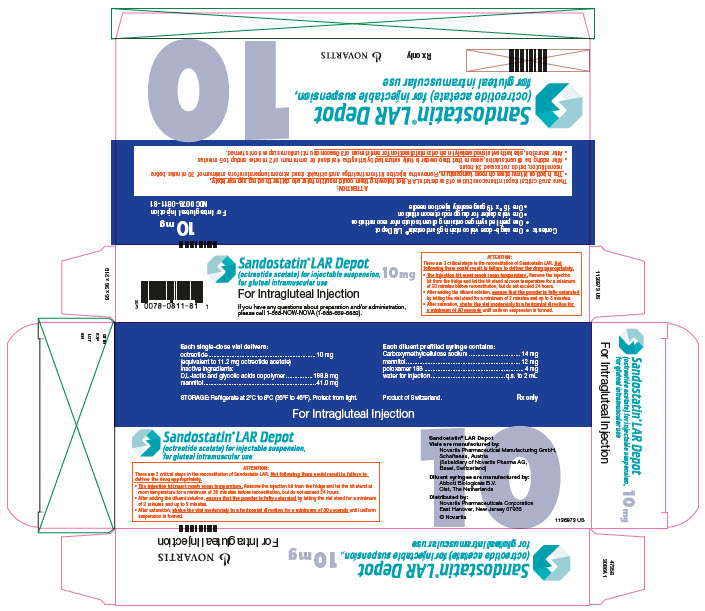
Sandostatin LAR Depot is available in a vial containing the sterile drug product, which when mixed with diluent, becomes a suspension that is given as a monthly intragluteal injection. The octreotide is uniformly distributed within the microspheres which are made of a biodegradable glucose star polymer, D,L-lactic and glycolic acids copolymer. Sterile mannitol is added to the microspheres to improve suspendability.
Sandostatin LAR Depot is available as: sterile 6-mL vials in 3 strengths delivering 10 mg, 20 mg, or 30 mg octreotide-free peptide. Each vial of Sandostatin LAR Depot delivers:
| *Equivalent to 10/20/30 mg octreotide base. | |||
| Name of Ingredient | 10 mg | 20 mg | 30 mg |
| octreotide acetate | 11.2 mg* | 22.4 mg* | 33.6 mg* |
| D,L-lactic and glycolic acids copolymer | 188.8 mg | 377.6 mg | 566.4 mg |
| mannitol | 41.0 mg | 81.9 mg | 122.9 mg |
| Each syringe of diluent contains: | |
| carboxymethylcellulose sodium | 14.0 mg |
| mannitol | 12.0 mg |
| poloxamer 188 | 4.0 mg |
| water for injection | 2.0 mL |
Sources
Sandostatin Lar Depot Manufacturers
-
Novartis Pharmaceuticals Corporation
![Sandostatin Lar Depot (Octreotide Acetate) Kit Sandostatin Lar Depot Demonstration Kit (Octreotide Acetate) Kit [Novartis Pharmaceuticals Corporation]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Sandostatin Lar Depot | Novartis Pharmaceuticals Corporation
![Sandostatin Lar Depot (Octreotide Acetate) Kit Sandostatin Lar Depot Demonstration Kit (Octreotide Acetate) Kit [Novartis Pharmaceuticals Corporation] Sandostatin Lar Depot (Octreotide Acetate) Kit Sandostatin Lar Depot Demonstration Kit (Octreotide Acetate) Kit [Novartis Pharmaceuticals Corporation]](/wp-content/themes/bootstrap/assets/img/loading2.gif)
Sandostatin LAR Depot should be administered by a trained healthcare provider. It is important to closely follow the mixing instructions included in the packaging. Sandostatin LAR Depot must be administered immediately after mixing.
Do not directly inject diluent without preparing suspension.
The recommended needle size for administration of Sandostatin LAR Depot is the 1½” 20 gauge safety injection needle (supplied in the drug product kit). For patients with a greater skin to muscle depth, a size 2” 20 gauge needle (not supplied) may be used.
Sandostatin LAR Depot should be administered intramuscularly in the gluteal region at 4-week intervals. Administration of Sandostatin LAR Depot at intervals greater than 4 weeks is not recommended.
Injection sites should be rotated in a systematic manner to avoid irritation. Deltoid injections should be avoided due to significant discomfort at the injection site when given in that area.
Sandostatin LAR Depot should never be administered intravenously or subcutaneously.The following dosage regimens are recommended.
2.1 AcromegalyPatients Not Currently Receiving Octreotide Acetate
Patients not currently receiving octreotide acetate should begin therapy with Sandostatin Injection given subcutaneously in an initial dose of 50 mcg three times daily which may be titrated. Most patients require doses of 100 mcg to 200 mcg three times daily for maximum effect but some patients require up to 500 mcg three times daily.
Patients should be maintained on Sandostatin Injection subcutaneous for at least 2 weeks to determine tolerance to octreotide. Patients who are considered to be “responders” to the drug, based on GH and IGF-1 levels and who tolerate the drug can then be switched to Sandostatin LAR Depot in the dosage scheme described below (Patients Currently Receiving Sandostatin Injection).
Patients Currently Receiving Sandostatin Injection
Patients currently receiving Sandostatin Injection can be switched directly to Sandostatin LAR Depot in a dose of 20 mg given IM intragluteally at 4-week intervals for 3 months. After 3 months, dosage may be adjusted as follows:
GH ≤2.5 ng/mL, IGF-1 normal, and clinical symptoms controlled: maintain Sandostatin LAR Depot dosage at 20 mg every 4 weeks.
GH >2.5 ng/mL, IGF-1 elevated, and/or clinical symptoms uncontrolled, increase Sandostatin LAR Depot dosage to 30 mg every 4 weeks.
GH ≤1 ng/mL, IGF-1 normal, and clinical symptoms controlled, reduce Sandostatin LAR Depot dosage to 10 mg every 4 weeks.
If GH, IGF-1, or symptoms are not adequately controlled at a dose of 30 mg, the dose may be increased to 40 mg every 4 weeks. Doses higher than 40 mg are not recommended.In patients who have received pituitary irradiation, Sandostatin LAR Depot should be withdrawn yearly for approximately 8 weeks to assess disease activity. If GH or IGF-1 levels increase and signs and symptoms recur, Sandostatin LAR Depot therapy may be resumed.
2.2 Carcinoid Tumors and VIPomasPatients Not Currently Receiving Octreotide Acetate
Patients not currently receiving octreotide acetate should begin therapy with Sandostatin Injection given subcutaneously. The suggested daily dosage for carcinoid tumors during the first 2 weeks of therapy ranges from 100-600 mcg/day in 2-4 divided doses (mean daily dosage is 300 mcg). Some patients may require doses up to 1500 mcg/day. The suggested daily dosage for VIPomas is 200-300 mcg in 2-4 divided doses (range 150-750 mcg); dosage may be adjusted on an individual basis to control symptoms but usually doses above 450 mcg/day are not required.
Sandostatin Injection should be continued for at least 2 weeks. Thereafter, patients who are considered “responders” to octreotide acetate and who tolerate the drug may be switched to Sandostatin LAR Depot in the dosage regimen as described below (Patients Currently Receiving Sandostatin Injection).
Patients Currently Receiving Sandostatin Injection
Patients currently receiving Sandostatin Injection can be switched to Sandostatin LAR Depot in a dosage of 20 mg given IM intragluteally at 4-week intervals for 2 months. Because of the need for serum octreotide to reach therapeutically effective levels following initial injection of Sandostatin LAR Depot, carcinoid tumor and VIPoma patients should continue to receive Sandostatin Injection subcutaneously for at least 2 weeks in the same dosage they were taking before the switch. Failure to continue subcutaneous injections for this period may result in exacerbation of symptoms. (Some patients may require 3 or 4 weeks of such therapy.)
After 2 months, dosage may be adjusted as follows:
If symptoms are adequately controlled, consider a dose reduction to 10 mg for a trial period. If symptoms recur, dosage should then be increased to 20 mg every 4 weeks. Many patients can, however, be satisfactorily maintained at a 10-mg dose every 4 weeks.
If symptoms are not adequately controlled, increase Sandostatin LAR Depot to 30 mg every 4 weeks. Patients who achieve good control on a 20-mg dose may have their dose lowered to 10 mg for a trial period. If symptoms recur, dosage should then be increased to 20 mg every 4 weeks.
Dosages higher than 30 mg are not recommended.Despite good overall control of symptoms, patients with carcinoid tumors and VIPomas often experience periodic exacerbation of symptoms (regardless of whether they are being maintained on Sandostatin Injection or Sandostatin LAR Depot). During these periods they may be given Sandostatin Injection subcutaneously for a few days at the dosage they were receiving prior to switching to Sandostatin LAR Depot. When symptoms are again controlled, the Sandostatin Injection subcutaneous can be discontinued.
2.3 Special Populations: Renal ImpairmentIn patients with renal failure requiring dialysis, the starting dose should be 10 mg every 4 weeks. In other patients with renal impairment, the starting dose should be similar to a nonrenal patient (i.e., 20 mg every 4 weeks) [see Clinical Pharmacology (12)].
2.4 Special Populations: Hepatic Impairment – Cirrhotic PatientsIn patients with established cirrhosis of the liver, the starting dose should be 10 mg every 4 weeks [see Clinical Pharmacology (12.3)].
Login To Your Free Account